Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
Click here to return to Chapter 8
| QUESTION | ANSWER |
|
1. Comment on the possible formation of the K2+ ion. Why is its formation unlikely? |
1. The K2+ ion is unlikely to form because the K+ ion already satisfies the octet rule and is rather stable. |
|
3. How many electrons does a Ba atom have to lose to have a complete octet in its valence shell? |
3. two |
|
5. How many electrons does an Se atom have to gain to have a complete octet in its valence shell? |
5. two |
|
7. With arrows, illustrate the transfer of electrons to form potassium chloride from K atoms and Cl atoms. |
7. 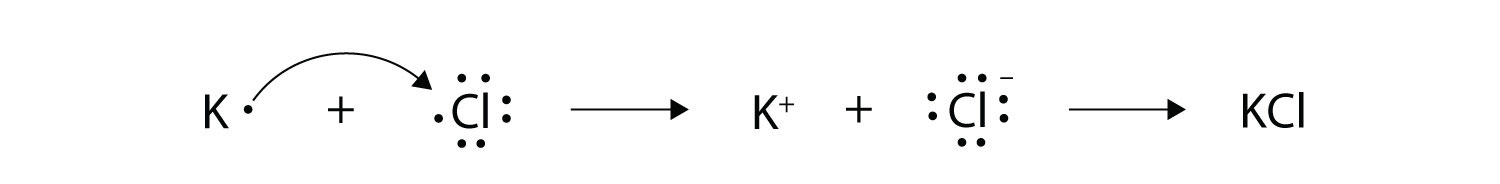 |
|
9. With arrows, illustrate the transfer of electrons to form scandium fluoride from Sc atoms and F atoms. |
9. 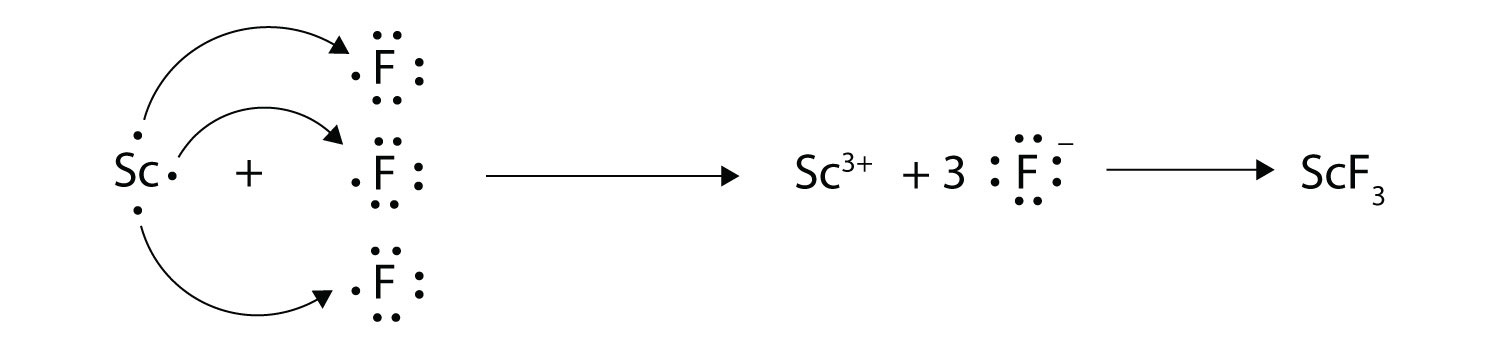 |
|
11. Which ionic compound has the higher lattice energy—KI or MgO? Why? |
11. MgO because the ions have a higher magnitude charge |
|
13. Which ionic compound has the higher lattice energy—BaS or MgO? Why? |
13. MgO because the ions are smaller |
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
