Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
Click here to return to Chapter 13
| QUESTION | ANSWER |
|
1. Cycloalkanes are named based on the number of C atoms in them, just like regular alkanes, but with the prefix cyclo- on the name. What are the names of the three smallest cycloalkanes? |
1. cyclopropane, cyclobutane, and cyclopentane |
|
3. Draw the carbon backbone of all noncyclic alkanes with only four C atoms. |
3.
 |
|
5. Cyclic alkanes can also have substituent groups on the ring. Draw the carbon backbone of all cyclic alkanes with only four C atoms. |
5.
|
|
7. Draw and name all possible isomers of pentene. |
7. |
|
9. Polyunsaturated alkenes have more than one C–C double bond. Draw the carbon backbone of all possible noncyclic polyunsaturated alkenes with four C atoms and two double bonds. What are the complete molecular formulas for each possible molecule? |
9. Both molecular formulas are C4H6. |
|
11, If a hydrocarbon is combined with enough halogen, all the H atoms will eventually be substituted with that halogen atom. Write the balanced chemical reaction between ethane and excess chlorine. |
11. C2H6 + 6Cl2 → C2Cl6 + 6HCl |
|
13. Molecules with multiple double bonds can also participate in addition reactions. Draw the structure of the product when butadiene, CH2=CH–CH=CH2, reacts with chlorine. |
13.
|
|
15. What is the maximum number of methyl groups that can be on a propane backbone before the molecule cannot be named as a propane compound? |
15. two |
|
17. In the gasoline industry, what is called isooctane is actually 2,2,4-trimethylpentane. Draw the structure of isooctane. |
17.
|
|
19. The actual name for the explosive TNT is 2,4,6-trinitrotoluene. If the structure of TNT is 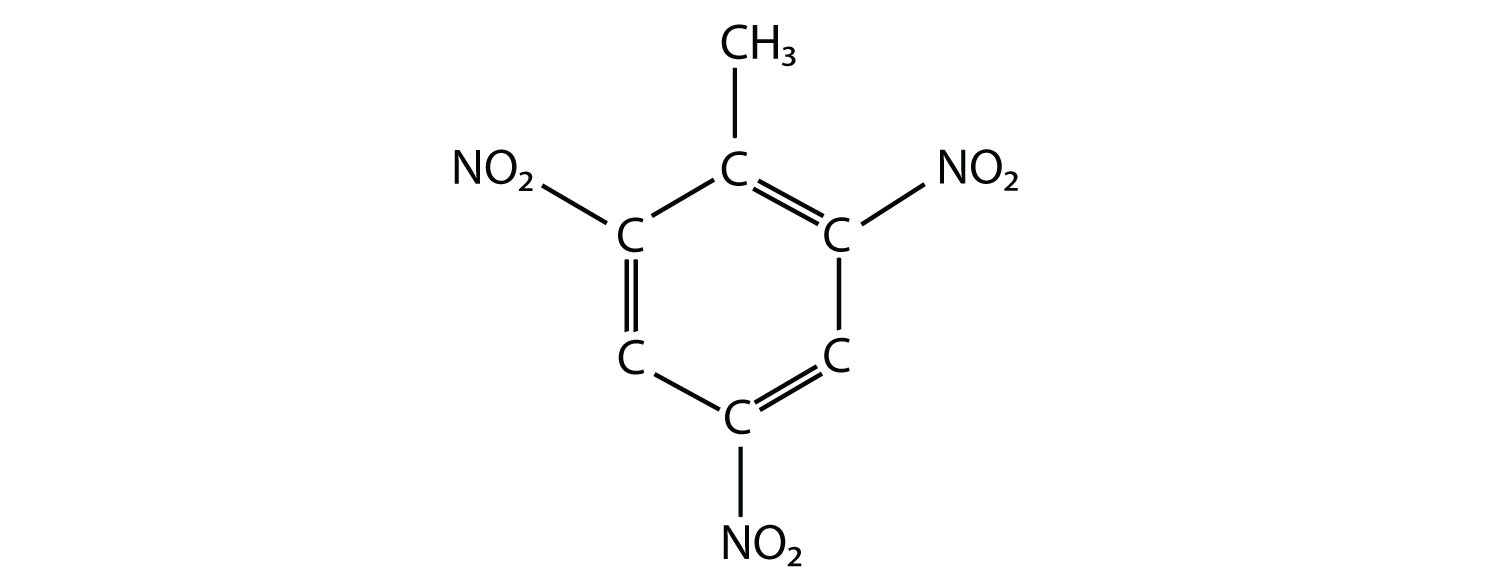 propose the structure of the parent compound toluene. |
19. 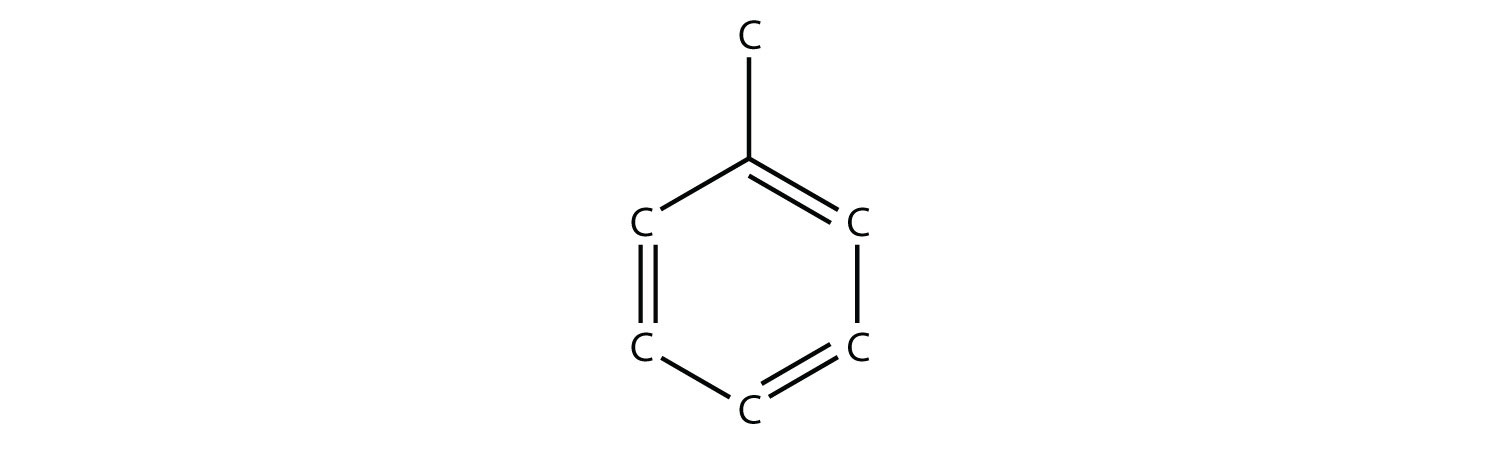 |
|
21. Draw the structures of all possible straight-chain isomers of bromopentane. |
21. 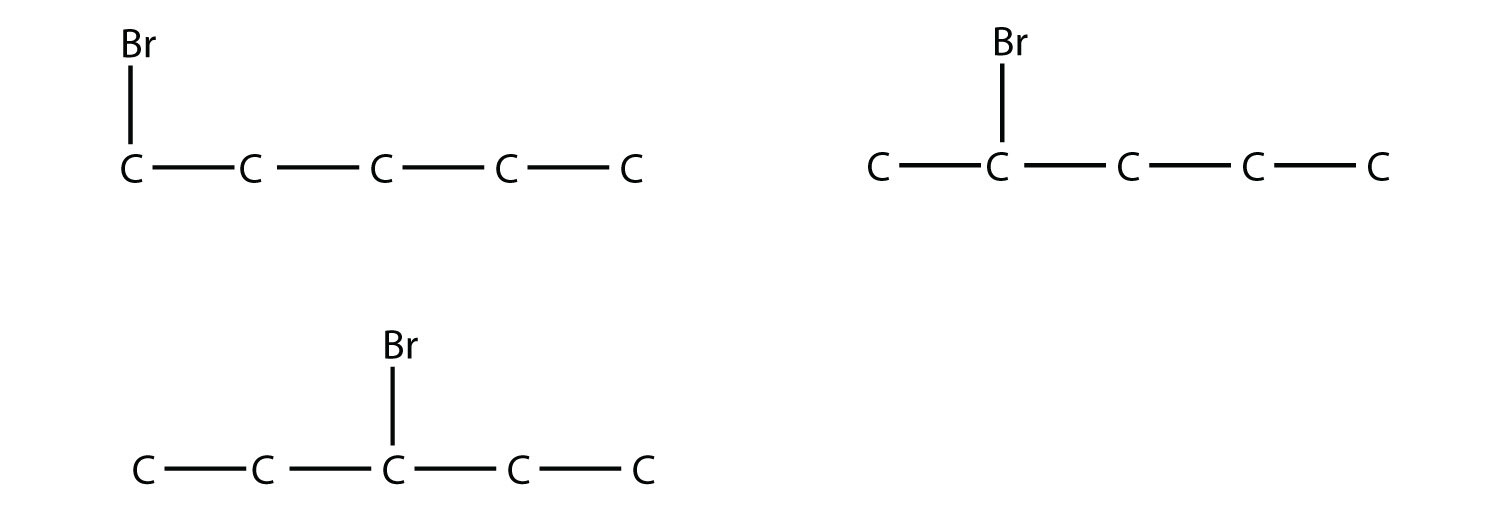 |
|
23. What is the final product of the double elimination of HCl from 1,1-dichloroethane? |
23. ethyne |
|
25. Draw the structure and name of the alcohol whose double elimination would yield the same product as in Exercise 23. Name the molecule as a hydroxyl-substituted compound. |
25. The names are 1,2-dihydroxyethane and 1,1-dihydroxyethane, respectively. |
|
27. Draw the smallest molecule that can have a separate aldehyde and carboxylic acid group. |
27.
|
|
29. Ethyl acetate is a common ingredient in nail-polish remover because it is a good solvent. Draw the structure of ethyl acetate. |
29. 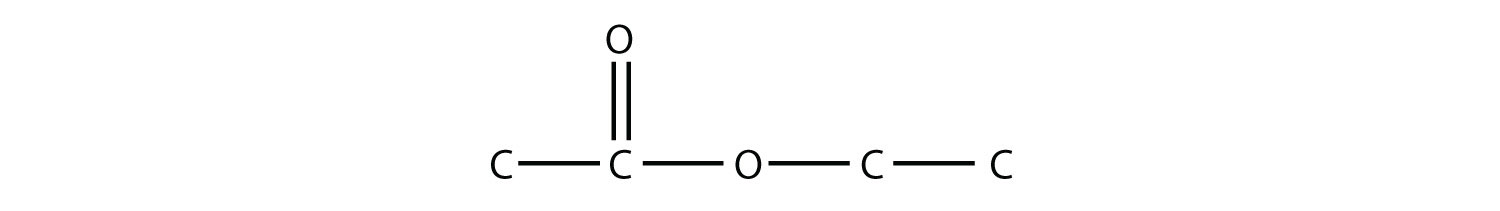 |
|
31. Draw the structure of diethyl ether, once used as an anesthetic. |
31. 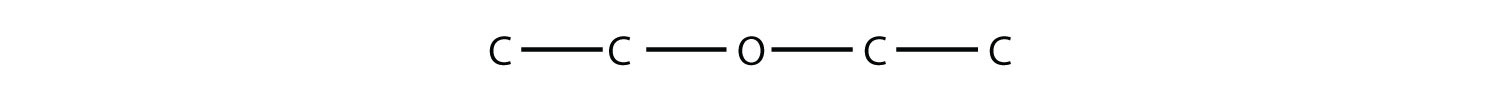 |
|
33. The odor of fish is caused by the release of small amine molecules, which vaporize easily and are detected by the nose. Lemon juice contains acids that react with the amines and make them not as easily vaporized, which is one reason why adding lemon juice to seafood is so popular. Write the chemical reaction of HCl with trimethylamine, an amine that is given off by seafood. |
33. (CH3)3N + HCl → (CH3)3NHCl |
|
35. With four monomers, draw two possible structures of a copolymer composed of ethylene and propylene. |
35. (answers will vary) 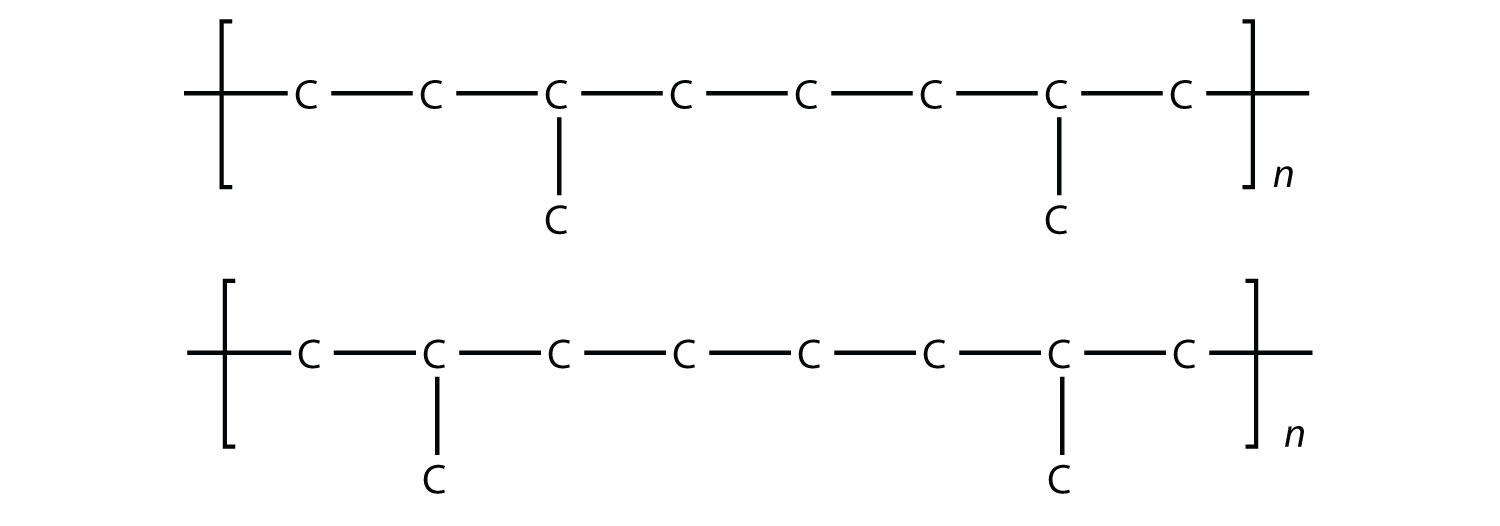 |
|
37. Draw the silicone that can be made from this monomer: 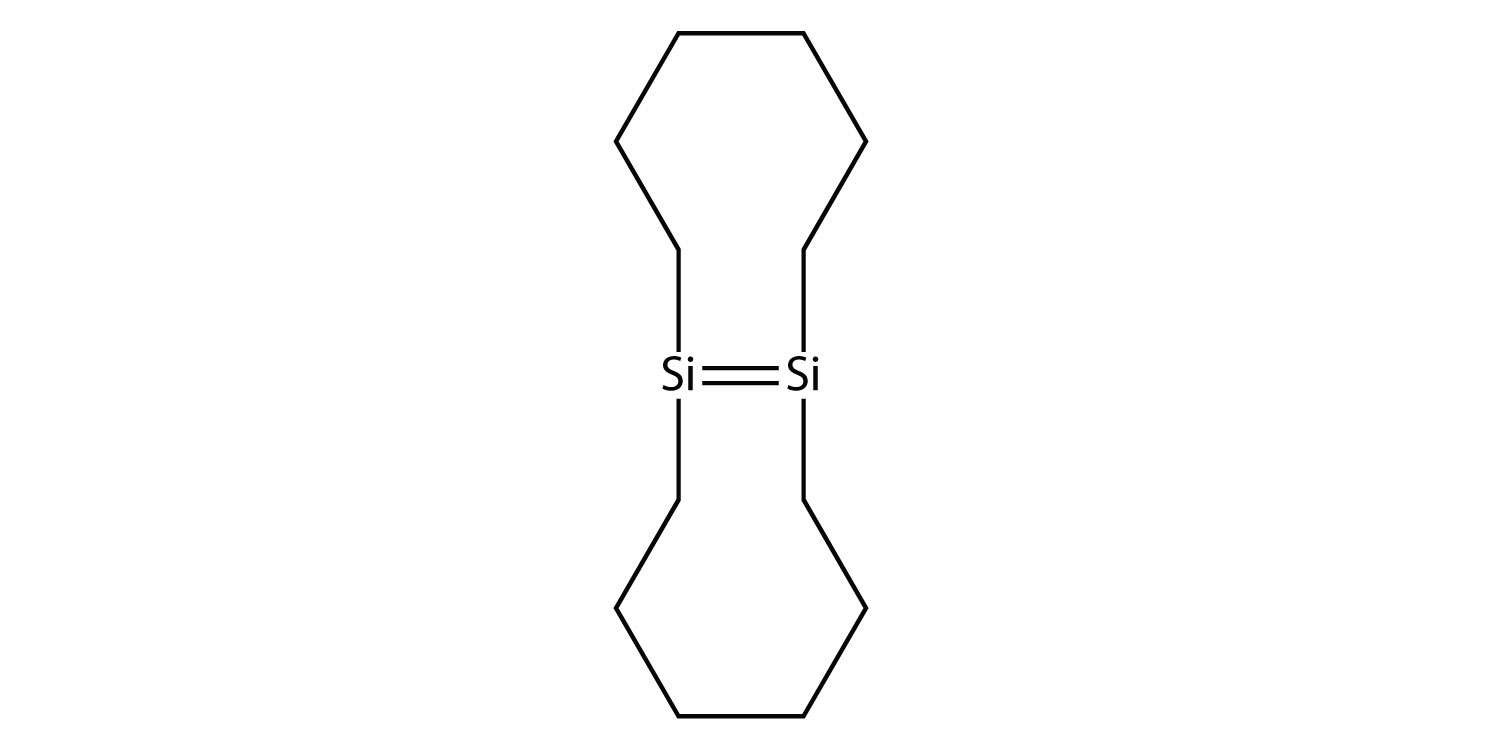 |
37. 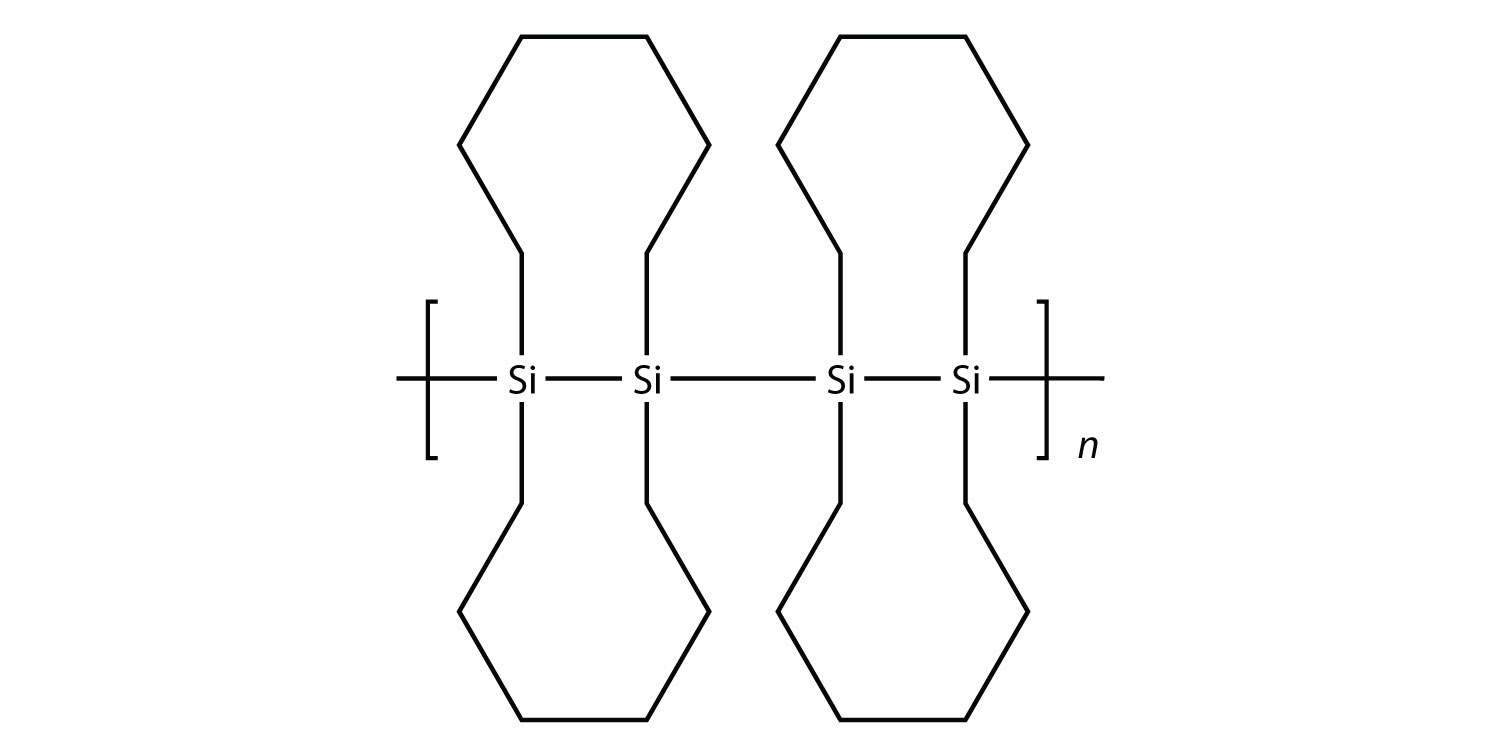 |
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
