Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
Click here to return to Chapter 13
| QUESTION | ANSWER |
|
1. Explain the relationship between a monomer and a polymer. |
1. A polymer is many monomers bonded together. |
|
3. Draw the polymer made from this monomer. 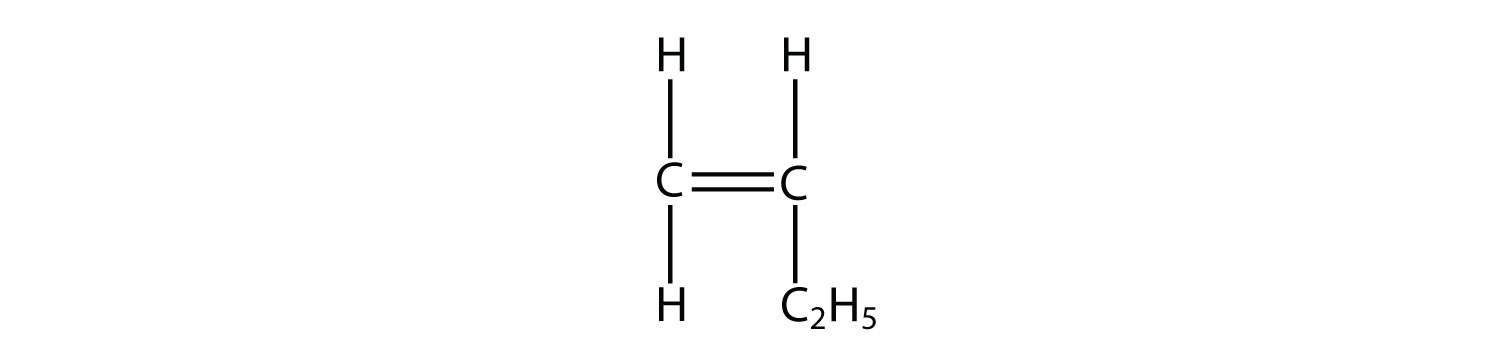 |
3.
|
|
5. What is the difference between an addition polymer and a condensation polymer? |
5. In an addition polymer, no small molecule is given off as a product, whereas in a condensation polymer, small parts of each monomer come off as a small molecule. |
|
7. List three properties of polymers that vary widely with composition. |
7. solubility in H2O and other solvents, melting point, flammability, color, hardness, transparency, film thickness, wetability, surface friction, moldability, and particle size (answers will vary) |
|
9. Draw the silicone made from this monomer. 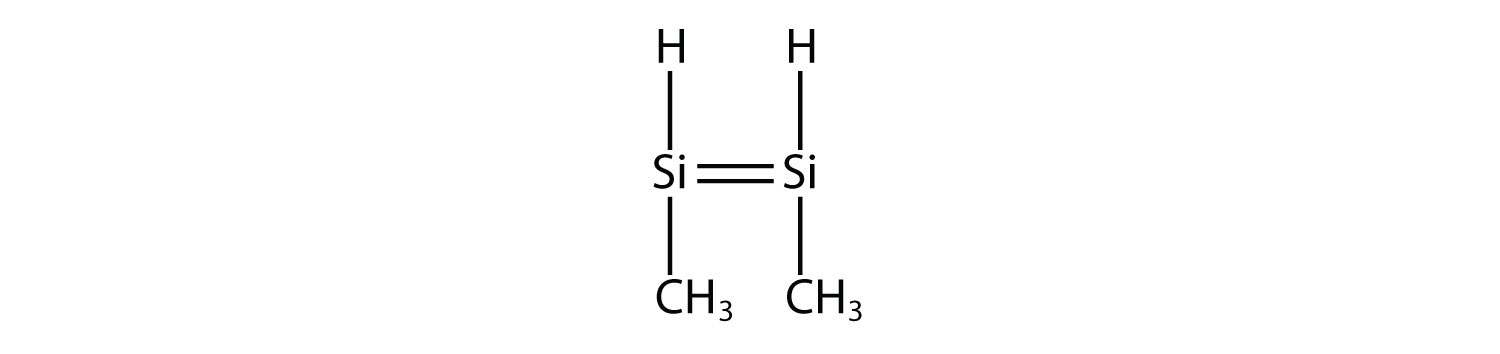 |
9.
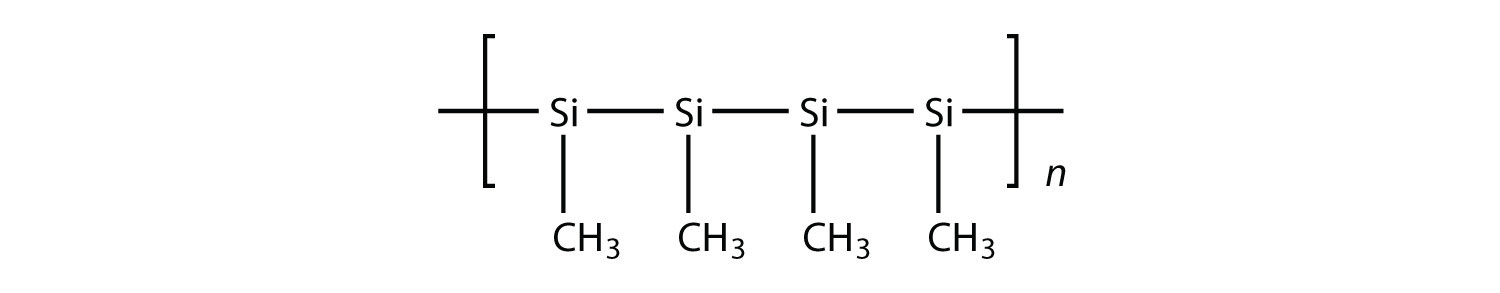 |
|
11. Explain how starch is a polymer. |
11. Starch is composed of many glucose monomer units. |
|
13. Explain how protein is a polymer. |
13. Proteins are polymers of amino acids, which act as the monomers. |
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
