Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
Click here to return to Chapter 13
| QUESTION | ANSWER |
|
1. Name a similarity between the functional groups found in aldehydes and ketones. Can you name a difference between them? |
1. They both have a carbonyl group, but an aldehyde has the carbonyl group at the end of a carbon chain, and a ketone has the carbonyl group in the middle. |
|
3. Name each molecule. |
3.
|
|
5. Name each molecule. |
5.
|
|
7. Name this molecule. 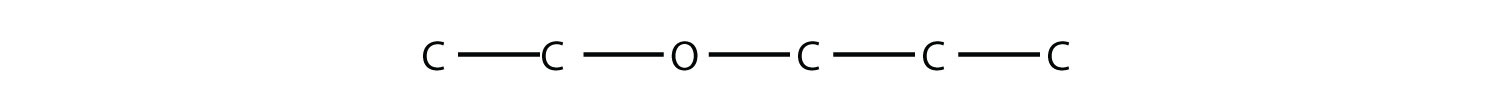 |
7. ethyl propyl ether |
|
9. Give an alternate but acceptable name to the molecule in Exercise 3b. |
9. ethyl methyl ketone |
|
11. Complete this chemical reaction. 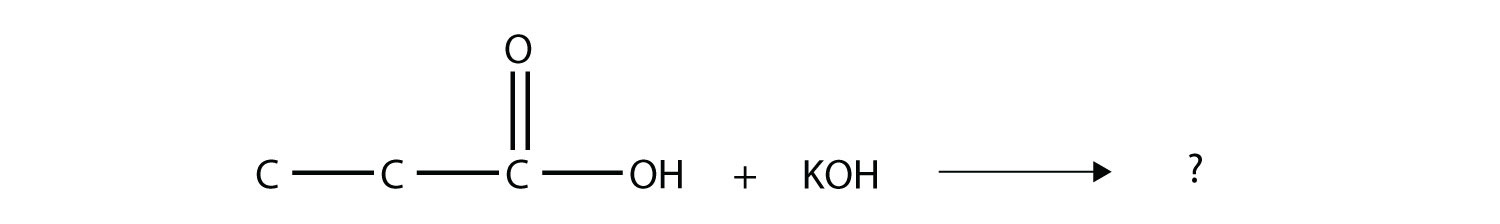 |
11. H2O + KCH3CH2CO2 |
|
13. The drug known as aspirin has this molecular structure: 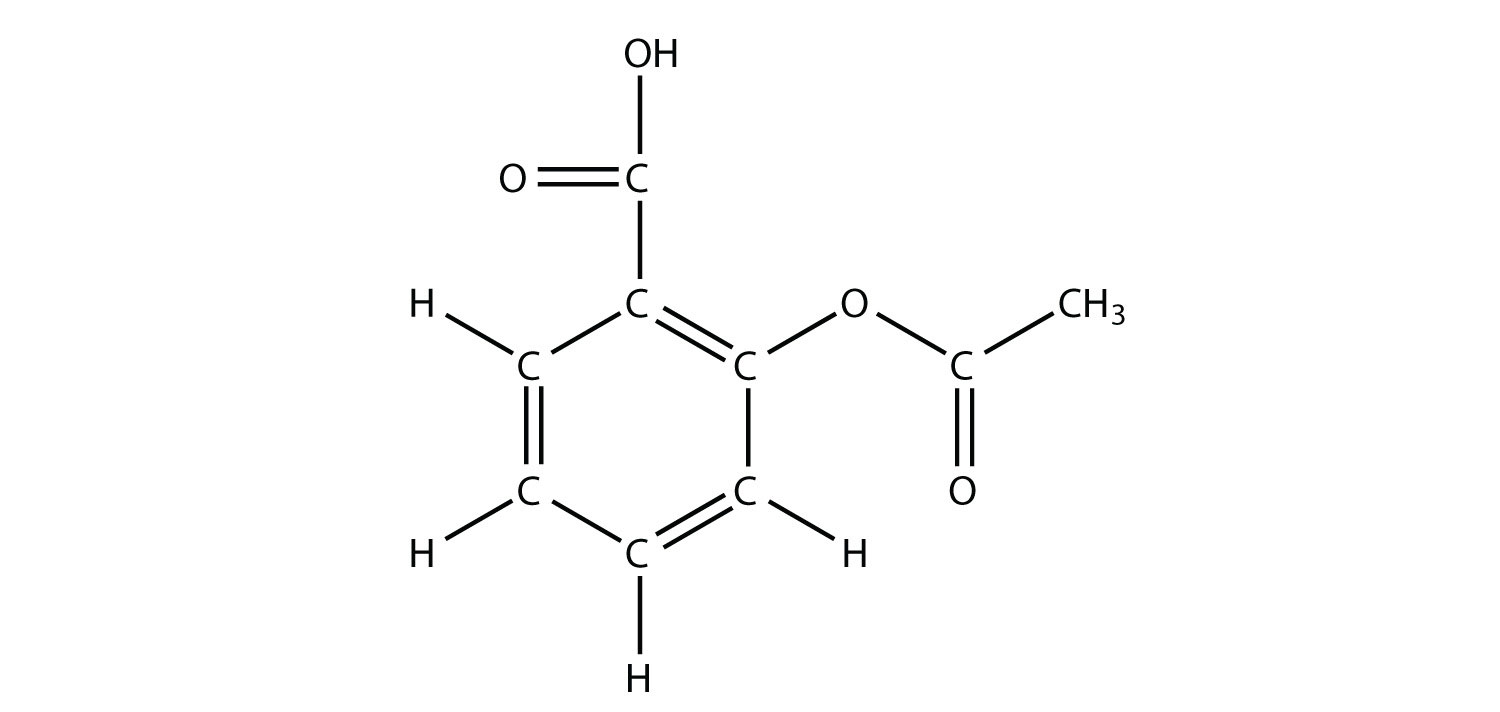 Identify the functional group(s) in this molecule. |
13. acid, ester, and aromatic (benzene ring) |
|
15. Identify the ester made by reacting these molecules. 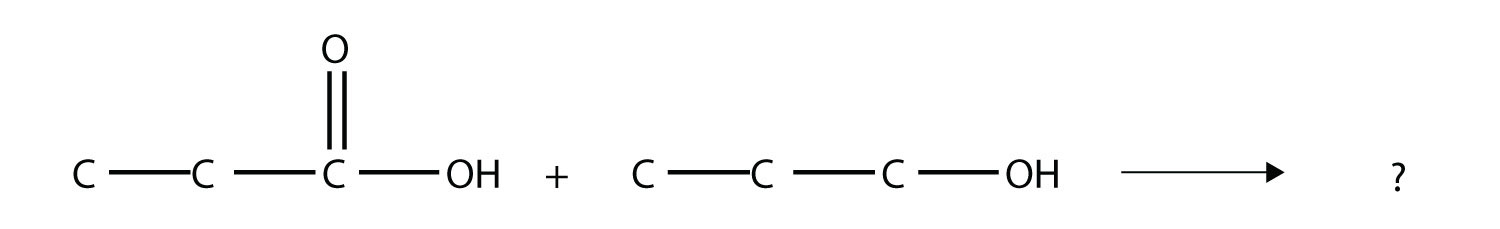 |
15. propyl propionate |
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
