Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
Click here to return to Chapter 8
| QUESTION | ANSWER |
|
1. How many electrons will be in the valence shell of H atoms when it makes a covalent bond? |
1. two |
|
3. What is the Lewis electron dot diagram of I2? Circle the electrons around each atom to verify that each valence shell is filled. |
3.
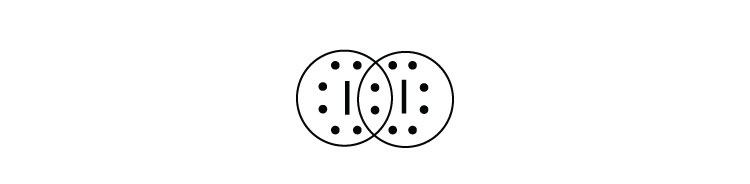 |
|
5. What is the Lewis electron dot diagram of NCl3? Circle the electrons around each atom to verify that each valence shell is filled. |
5. 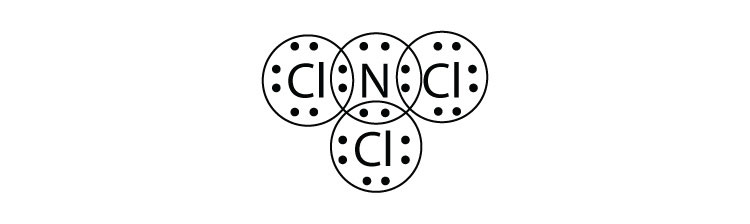 |
|
7. Draw the Lewis electron dot diagram for each substance.
|
7. |
|
9. Draw the Lewis electron dot diagram for each substance.
|
9. |
|
11. Draw the Lewis electron dot diagram for each substance. Double or triple bonds may be needed.
|
11. |
|
13. Draw the Lewis electron dot diagram for each substance. Double or triple bonds may be needed.
|
13. |
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
