Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball, John W. Hill, and Rhonda J. Scott
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
Amines are classified according to the number of carbon atoms bonded directly to the nitrogen atom. A primary (1°) amine has one alkyl (or aryl) group on the nitrogen atom, a secondary (2°) amine has two, and a tertiary (3°) amine has three (Figure 5.1 "The Structure of Amines Compared to Water, an Alcohol, and an Ether").
Figure 5.1 The Structure of Amines Compared to Water, an Alcohol, and an Ether
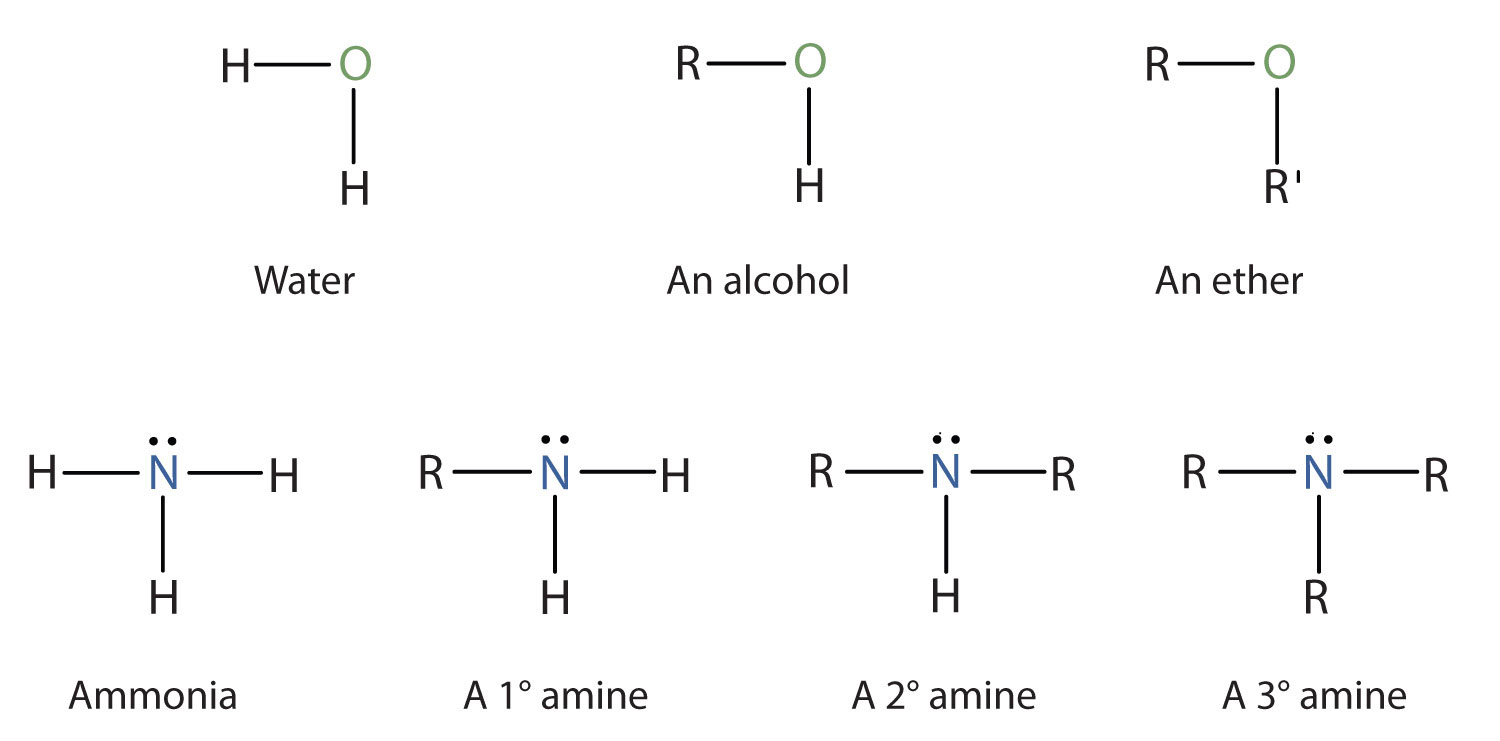
To classify alcohols, we look at the number of carbon atoms bonded to the carbon atom bearing the OH group, not the oxygen atom itself. Thus, although isopropylamine looks similar to isopropyl alcohol, the former is a primary amine, while the latter is a secondary alcohol.

The common names for simple aliphatic amines consist of an alphabetic list of alkyl groups attached to the nitrogen atom, followed by the suffix -amine. (Systematic names are often used by some chemists.) The amino group (NH2) is named as a substituent in more complicated amines, such as those that incorporate other functional groups or in which the alkyl groups cannot be simply named.
Name and classify each compound.
1. CH3CH2CH2NH2
2.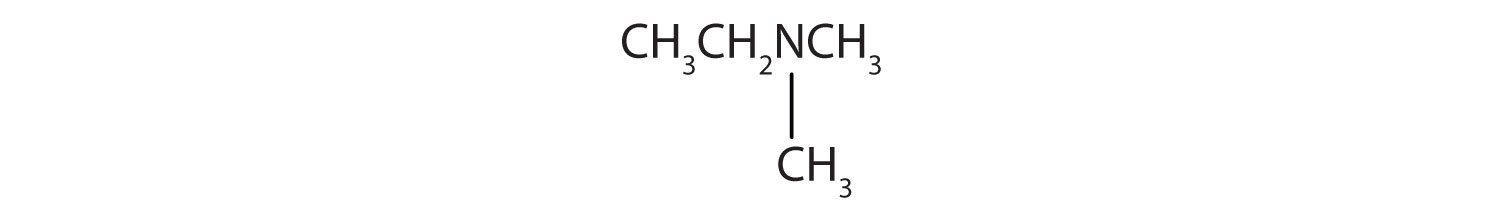
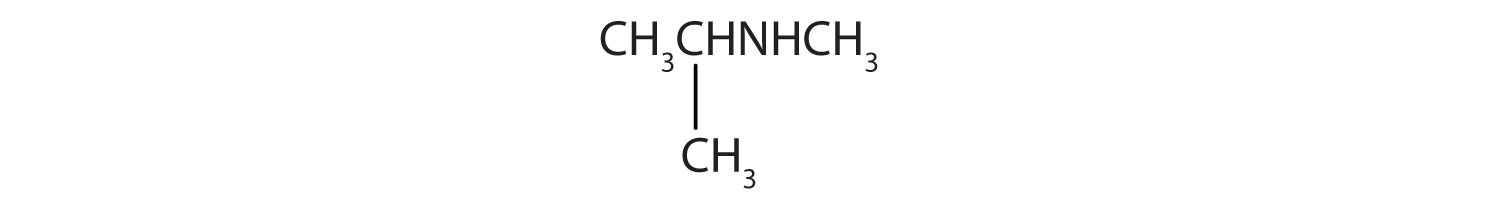
3. CH3CH2NHCH2CH3
4. CH3CH2CH2NHCH3
Solution
Name and classify each compound.
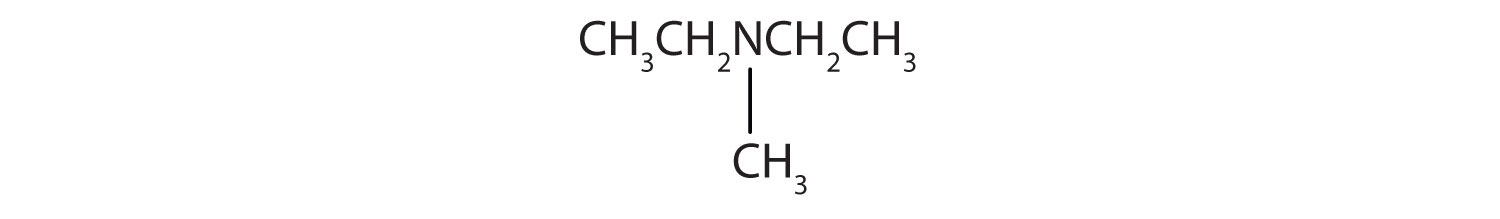
CH3CH2CH2CH2NH2
CH3CH2CH2NHCH2CH2 CH3
Draw the structure for each compound and classify.
Solution
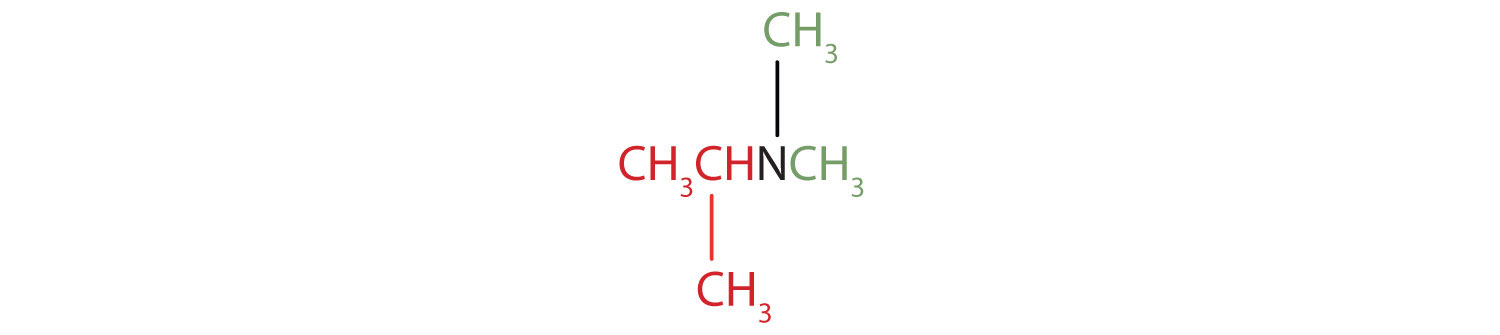
1. The name indicates that there are an isopropyl group (in red) and two methyl groups (in green) attached to the nitrogen atom; the amine is tertiary.
.
2. The name indicates that there are two propyl groups attached to the nitrogen atom; the amine is secondary. (The third bond on the nitrogen atom goes to a hydrogen atom.)
CH3CH2CH2NHCH2CH2CH3
Draw the structure for each compound and classify.
ethylisopropylamine
diethylpropylamine
The primary amine in which the nitrogen atom is attached directly to a benzene ring has a special name—aniline. Aryl amines are named as derivatives of aniline.
Name this compound.
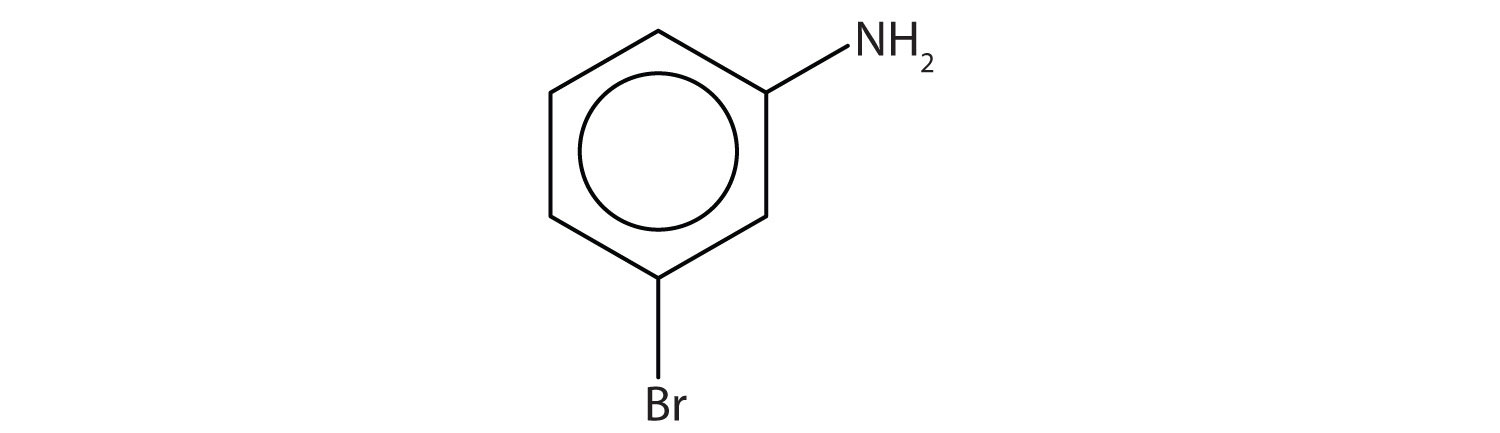
Solution
The benzene ring with an amino (NH2) group is aniline. The compound is named as a derivative of aniline: 3-bromoaniline or m-bromoaniline.
Name this compound.
Draw the structure for p-ethylaniline and classify.
Solution
The compound is a derivative of aniline. It is a primary amine having an ethyl group located para to the amino (NH2) group.
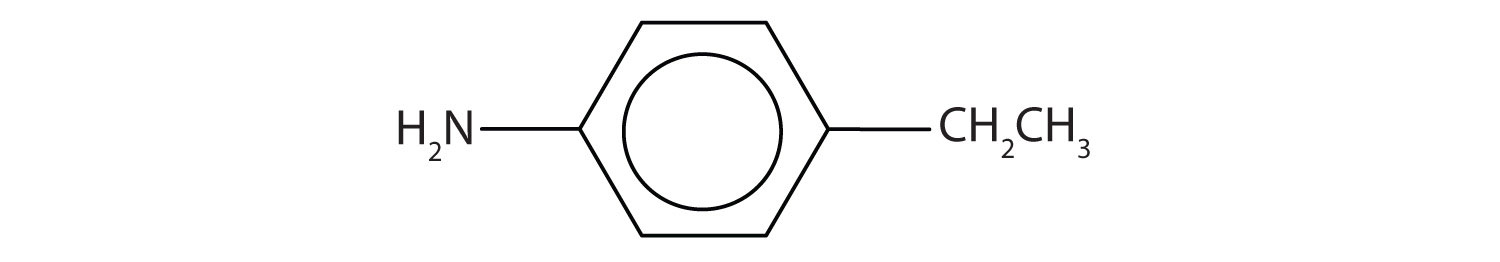
Draw the structure for p-isopropylaniline and classify.
Draw the structure for 2-amino-3-methylpentane.
Solution
Always start with the parent compound: draw the pentane chain. Then attach a methyl group at the third carbon atom and an amino group at the second carbon atom.
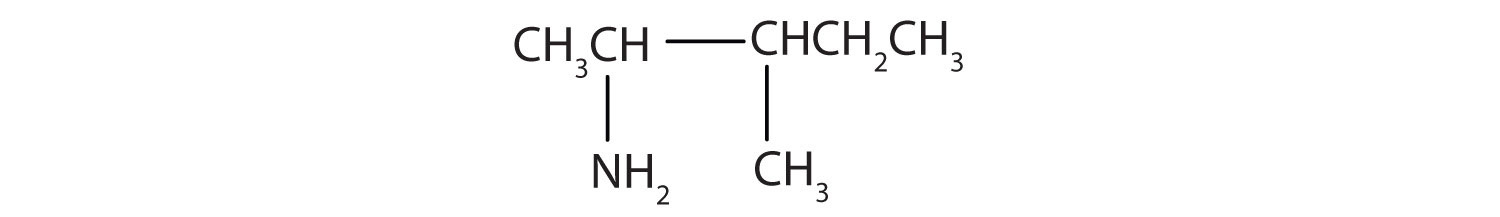
Draw the structure for 2-amino-3-ethyl-1-chloroheptane.
Ammonium (NH4+) ions, in which one or more hydrogen atoms are replaced with alkyl groups, are named in a manner analogous to that used for simple amines. The alkyl groups are named as substituents, and the parent species is regarded as the NH4+ ion. For example, CH3NH3+ is the methylammonium ion. The ion formed from aniline (C6H5NH3+) is called the anilinium ion.
Name each ion.
Solution
The ions have one, two, three, and four methyl (CH3) groups attached to a nitrogen atom. Their names are as follows:
Name each ion.
CH3CH2NH3+
(CH3CH2)3NH+
(CH3CH2CH2)2NH2+
(CH3CH2CH2CH2)4N+
To what inorganic compound are the amines related?
How are amines classified?
ammonia
by the number of hydrocarbon groups on the nitrogen atom: primary amine, one group; secondary amine, two groups; tertiary amine, three groups
The amine functional group is as follows:
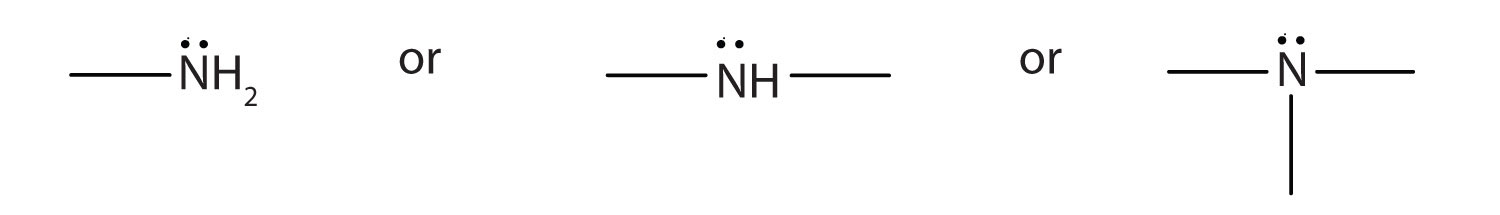
1. Draw the structure for each compound and classify the amine as primary, secondary, or tertiary.
a. dimethylamine
b. diethylmethylamine
c. 2-aminoethanol
2. Draw the structure for each compound and classify the amine as primary, secondary, or tertiary.
a. 3-aminopentane
b. 1,6-diaminohexane
c. ethylphenylamine
3. Draw the structure for each compound.
a. aniline
b. m-bromoaniline
4. Draw the structure for each compound.
a. 2-chloroaniline
b. 3,5-dichloroaniline
5. Name each compound.
a. CH3CH2CH2NH2
b.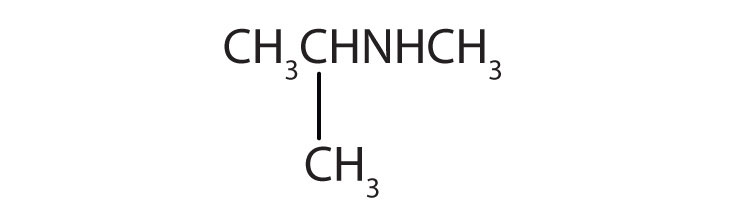
c.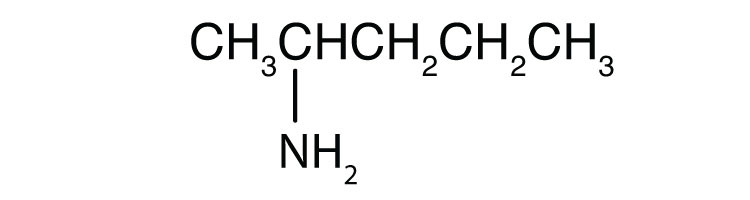
6. Name each compound.
a. (CH3CH2)3N
b. (CH3CH2)2NCH3
7. Draw the structure for each compound.
a. dimethylammonium chloride
b. anilinium chloride
8. Draw the structure for each compound.
a. ethylmethylammonium chloride
b. anilinium nitrate
9. Name each compound.
a. [CH3CH2NH2CH2CH3]+Br−
b. [(CH3CH2)3NH]+I−
10. Name each compound.
a. [(CH3)3NH]+NO3−
b. [(CH3CH2)2NH2]+Cl−
1.
a. CH3NHCH3; secondary
b. tertiary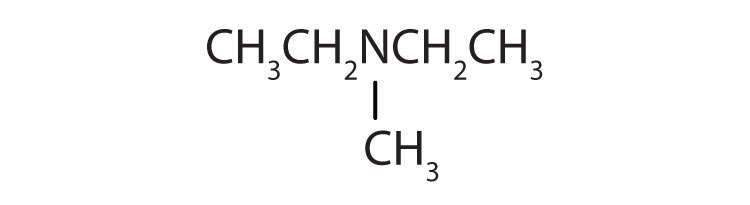
c. HOCH2CH2NH2; primary
3.
a.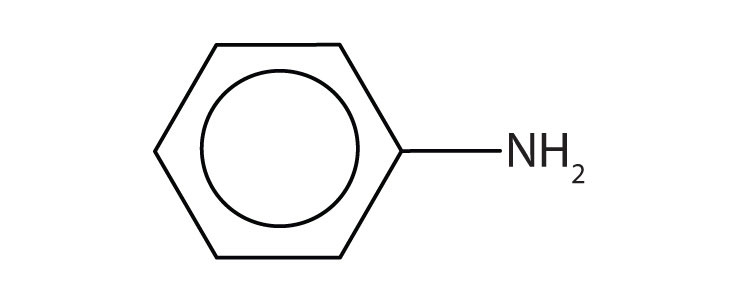
b.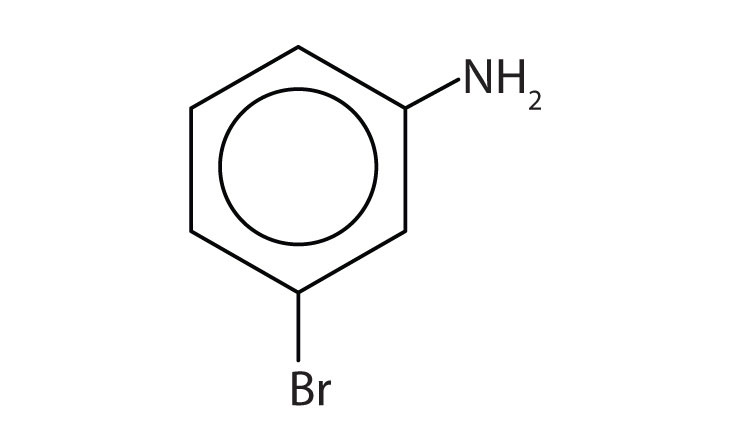
5.
a. propylamine
b. isopropylmethylamine
c. 2-aminopentane
7.
a. [(CH3)2NH2+]Cl–
b.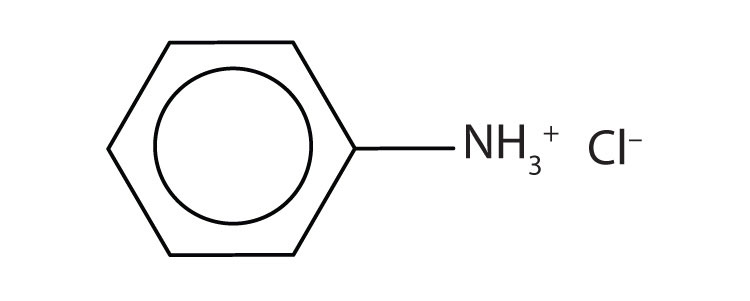
9.
a. diethylammonium bromide
b. triethylammonium iodide
Primary and secondary amines have hydrogen atoms bonded to an nitrogen atom and are therefore capable of hydrogen bonding (part (a) of Figure 5.2 "Hydrogen Bonding"), although not as strongly as alcohol molecules (which have hydrogen atoms bonded to an oxygen atom, which is more electronegative than nitrogen). These amines boil at higher temperatures than alkanes but at lower temperatures than alcohols of comparable molar mass. For example, compare the boiling point of methylamine (CH3NH2; −6°C) with those of ethane (CH3CH3; −89°C) and methanol (CH3OH; 65°C). Tertiary amines have no hydrogen atom bonded to the nitrogen atom and so cannot participate in intermolecular hydrogen bonding. They have boiling points comparable to those of ethers (Table 5.1 "Physical Properties of Some Amines and Comparable Oxygen-Containing Compounds").
Figure 5.2 Hydrogen Bonding
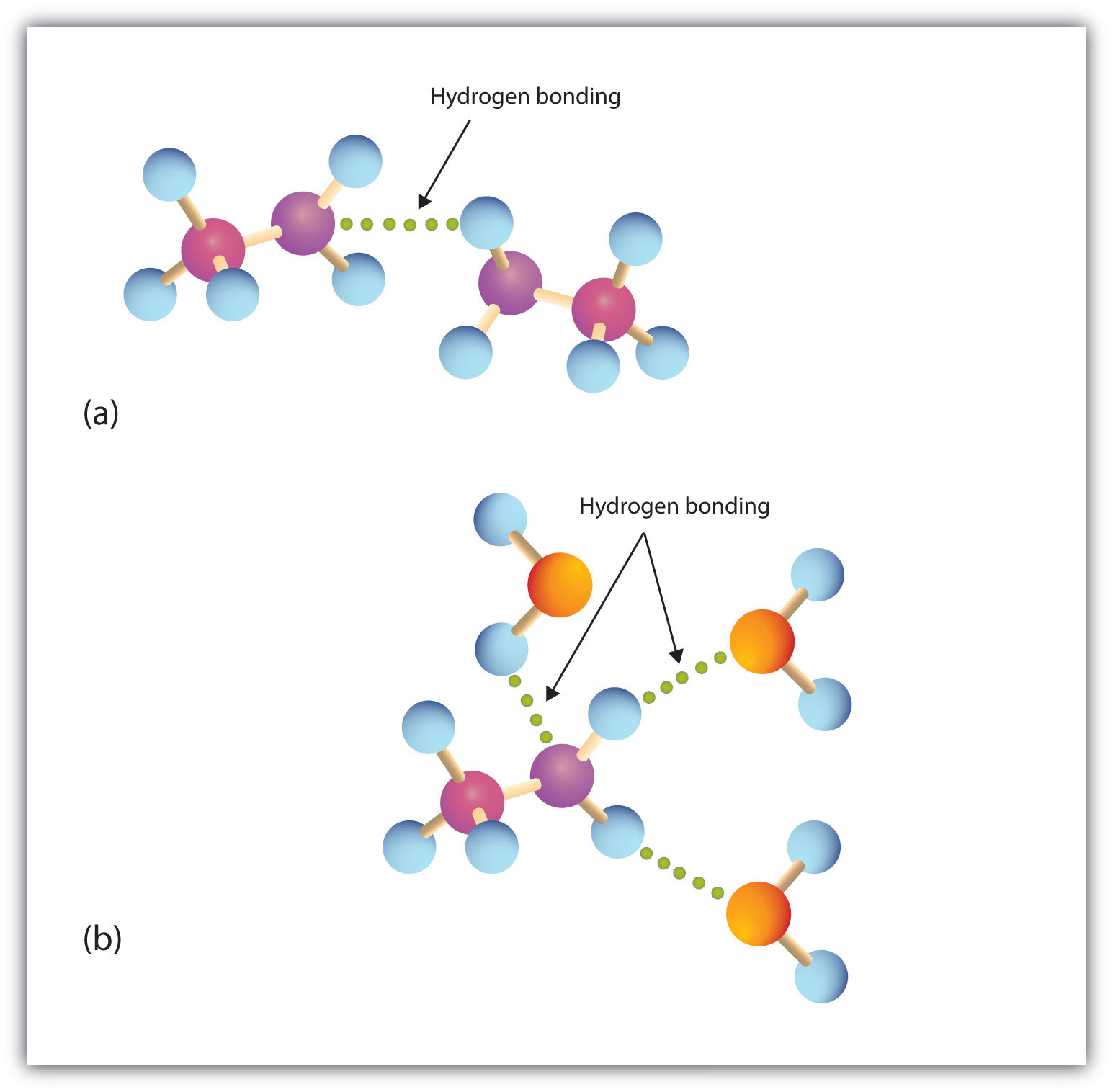
(a) Amine molecules are associated through hydrogen bonding. (b) An amine molecule can form a hydrogen bond with water molecules.
Table 5.1 Physical Properties of Some Amines and Comparable Oxygen-Containing Compounds
| Name | Condensed Structural Formula | Class | Molar Mass | Boiling Point (°C) | Solubility at 25°C (g/100 g Water) |
|---|---|---|---|---|---|
| butylamine | CH3CH2CH2CH2NH2 | 1° | 73 | 78 | miscible |
| diethylamine | (CH3CH2)2NH | 2° | 73 | 55 | miscible |
| butyl alcohol | CH3CH2CH2CH2OH | — | 74 | 118 | 8 |
| dipropylamine | (CH3CH2CH2)2NH | 2° | 101 | 111 | 4 |
| triethylamine | (CH3CH2)3N | 3° | 101 | 90 | 14 |
| dipropyl ether | (CH3CH2CH2)2O | — | 102 | 91 | 0.25 |
All three classes of amines can engage in hydrogen bonding with water (part (b) of Figure 5.2 "Hydrogen Bonding"). Amines of low molar mass are quite soluble in water; the borderline of solubility in water is at five or six carbon atoms.
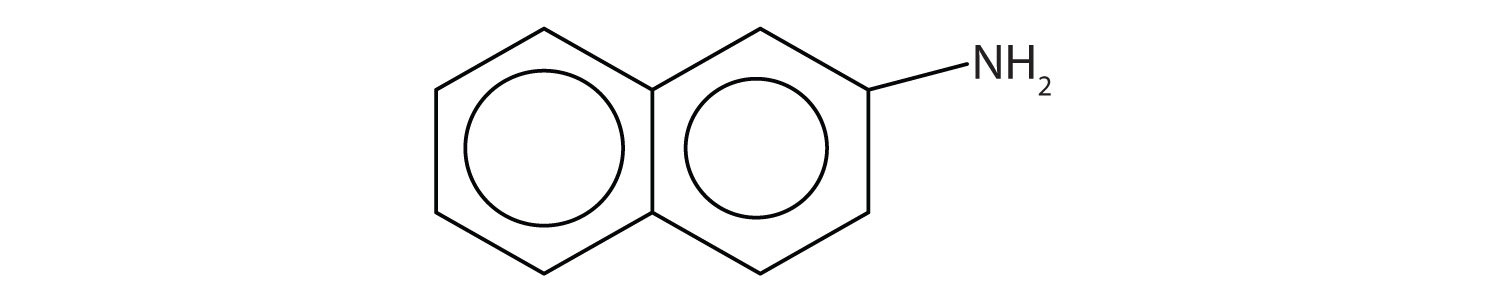
Amines have “interesting” odors. The simple ones smell very much like ammonia. Higher aliphatic amines smell like decaying fish. Or perhaps we should put it the other way around: Decaying fish give off odorous amines. The stench of rotting fish is due in part to two diamines: putrescine and cadaverine. They arise from the decarboxylation of ornithine and lysine, respectively, amino acids that are found in animal cells. (For more information about lysine, see Chapter 8 "Amino Acids", Section 8.1 "Properties of Amino Acids".)
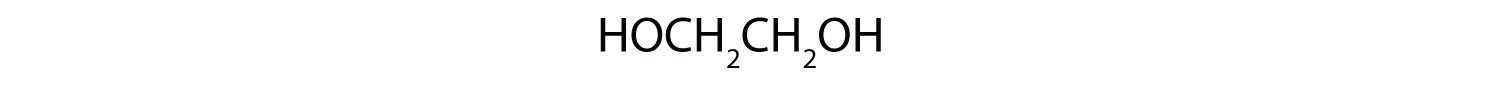
Aromatic amines generally are quite toxic. They are readily absorbed through the skin, and workers must exercise caution when handling these compounds. Several aromatic amines, including β-naphthylamine, are potent carcinogens.
Which compound has the higher boiling point, CH3CH2CH2CH2CH2NH2 or CH3CH2CH2CH2CH2CH3? Explain.
Which compound is more soluble in water, CH3CH2CH2CH2CH3 or CH3CH2NHCH2CH3? Explain.
CH3CH2CH2CH2CH2NH2 because the nitrogen-to-hydrogen (N–H) bonds can engage in hydrogen bonding; CH3CH2CH2CH2CH2CH3 cannot engage in hydrogen bonding
CH3CH2NHCH2CH3 because amines can engage in hydrogen bonding with water; alkanes cannot engage in hydrogen bonding
1. Which compound of each pair has the higher boiling point? Explain.
a. butylamine or pentane
b. CH3NH2 or CH3CH2CH2CH2CH2NH2
2. Which compound of each pair has the higher boiling point? Explain.
a. butylamine or butyl alcohol
b. trimethylamine or propylamine
3. Which compound is more soluble in water—CH3CH2CH3 or CH3CH2NH2? Explain.
4. Which compound is more soluble in water—CH3CH2CH2NH2 or CH3CH2CH2CH2CH2CH2NH2? Explain.
1.
a. butylamine because the N–H bonds can engage in hydrogen bonding; pentane cannot engage in hydrogen bonding
b. CH3CH2CH2CH2CH2NH2 because it has a greater molar mass than CH3NH2
3. CH3CH2NH2 because amines can engage in hydrogen bonding with water; alkanes cannot engage in hydrogen bonding
Recall that ammonia (NH3) acts as a base because the nitrogen atom has a lone pair of electrons that can accept a proton. Amines also have a lone electron pair on their nitrogen atoms and can accept a proton from water to form substituted ammonium (NH4+) ions and hydroxide (OH−) ions:
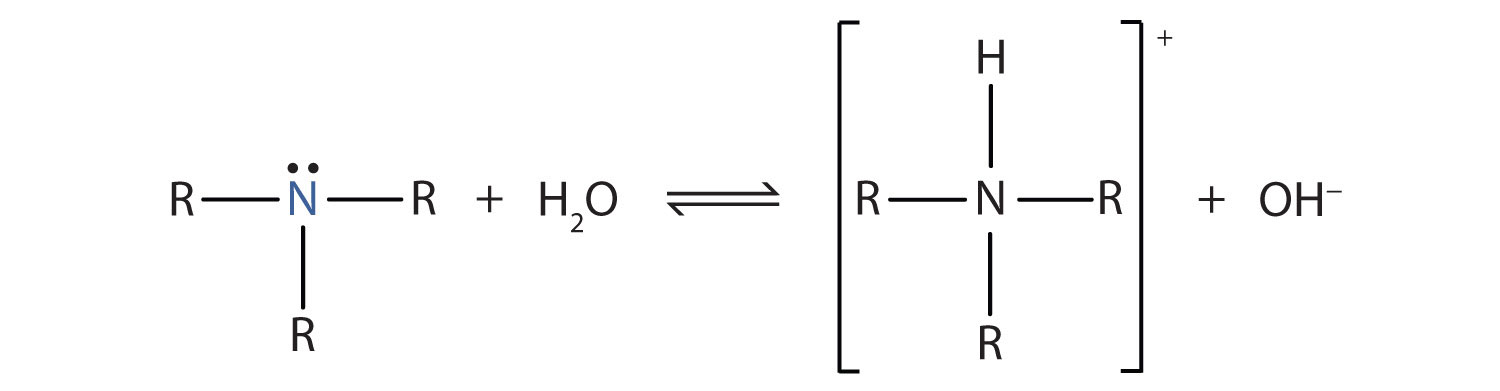
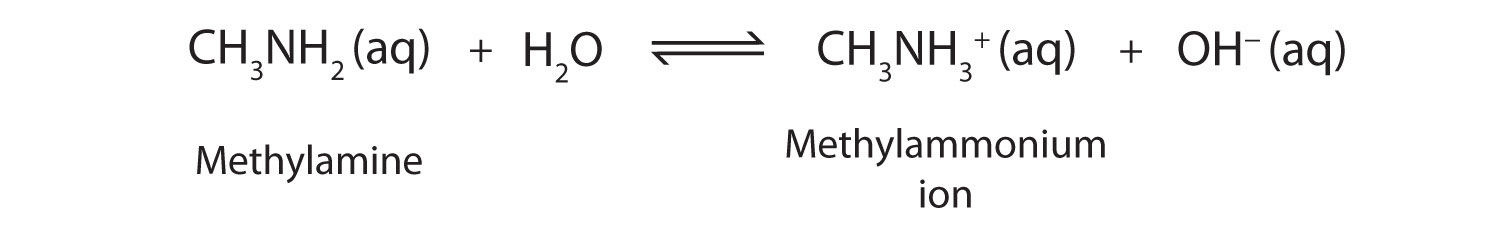
As a specific example, methylamine reacts with water to form the methylammonium ion and the OH− ion.
Nearly all amines, including those that are not very soluble in water, will react with strong acids to form salts soluble in water.
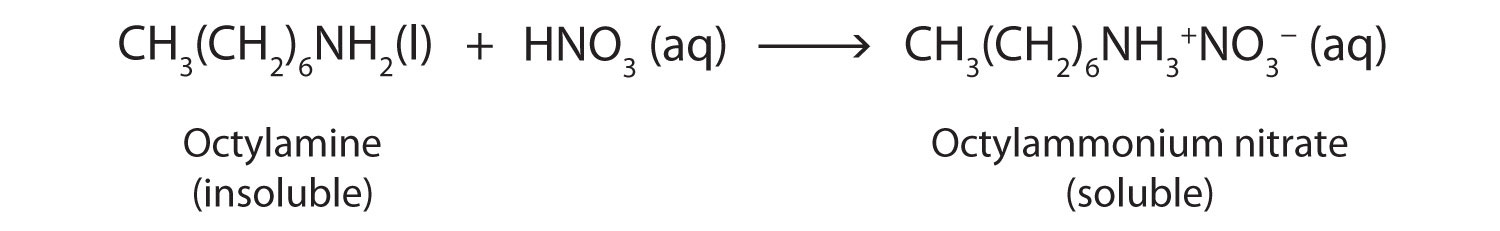
Amine salts are named like other salts: the name of the cation is followed by the name of the anion.
What are the formulas of the acid and base that react to form [CH3NH2CH2CH3]+CH3COO−?
Solution
The cation has two groups—methyl and ethyl—attached to the nitrogen atom. It comes from ethylmethylamine (CH3NHCH2CH3). The anion is the acetate ion. It comes from acetic acid (CH3COOH).
What are the formulas of the acid and base that react to form (CH3CH2CH2)3NH+I−?
Salts of aniline are properly named as anilinium compounds, but an older system, still in use for naming drugs, identifies the salt of aniline and hydrochloric acid as “aniline hydrochloride.” These compounds are ionic—they are salts—and the properties of the compounds (solubility, for example) are those characteristic of salts. Many drugs that are amines are converted to hydrochloride salts to increase their solubility in aqueous solution.
Looking back at the various cyclic hydrocarbons introduced in Chapter 1 "Organic Chemistry Review / Hydrocarbons", we see that all the atoms in the rings of these compounds are carbon atoms. In other cyclic compounds, called heterocyclic compounds (Greek heteros, meaning “other”), nitrogen, oxygen, sulfur, or some other atom is incorporated in the ring. Many heterocyclic compounds are important in medicine and biochemistry. Some compose part of the structure of the nucleic acids, which in turn compose the genetic material of cells and direct protein synthesis. (For more information about nucleic acids, see Chapter 10 "Nucleic Acids and Protein Synthesis".)
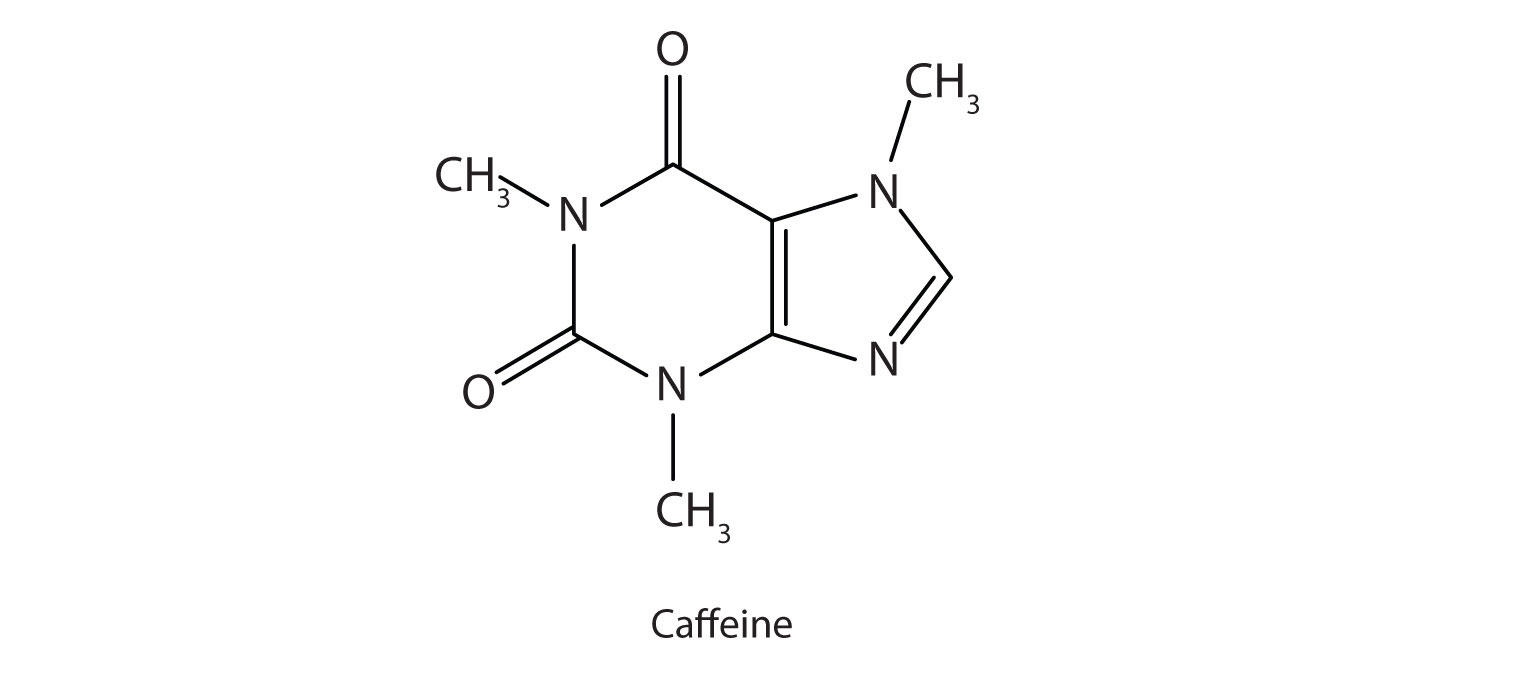
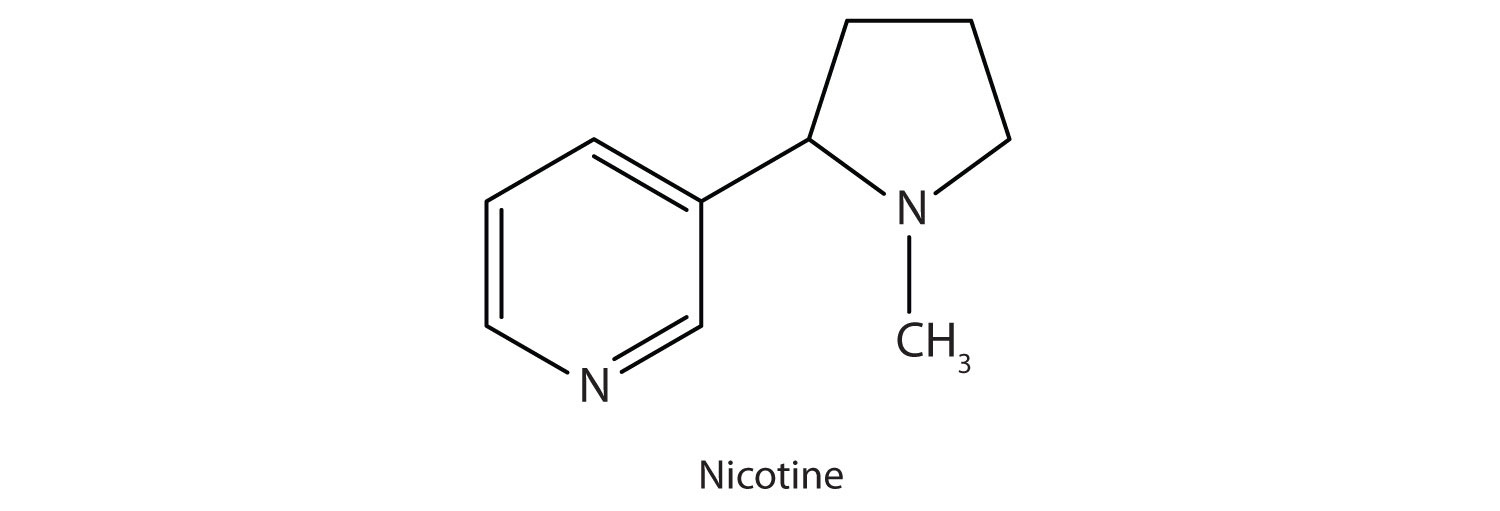
Many heterocyclic amines occur naturally in plants. Like other amines, these compounds are basic. Such a compound is an alkaloid, a name that means “like alkalis.” Many alkaloids are physiologically active, including the familiar drugs caffeine, nicotine, and cocaine.
Caffeine is a stimulant found in coffee, tea, and some soft drinks. Its mechanism of action is not well understood, but it is thought to block the activity of adenosine, a heterocyclic base that acts as a neurotransmitter, a substance that carries messages across a tiny gap (synapse) from one nerve cell (neuron) to another cell. The effective dose of caffeine is about 200 mg, corresponding to about two cups of strong coffee or tea.
Nicotine acts as a stimulant by a different mechanism; it probably mimics the action of the neurotransmitter acetylcholine. People ingest this drug by smoking or chewing tobacco. Its stimulant effect seems transient, as this initial response is followed by depression. Nicotine is highly toxic to animals. It is especially deadly when injected; the lethal dose for a human is estimated to be about 50 mg. Nicotine has also been used in agriculture as a contact insecticide.
Cocaine acts as a stimulant by preventing nerve cells from taking up dopamine, another neurotransmitter, from the synapse. High levels of dopamine are therefore available to stimulate the pleasure centers of the brain. The enhancement of dopamine action is thought to be responsible for cocaine’s “high” and its addictive properties. After the binge, dopamine is depleted in less than an hour. This leaves the user in a pleasureless state and (often) craving more cocaine.
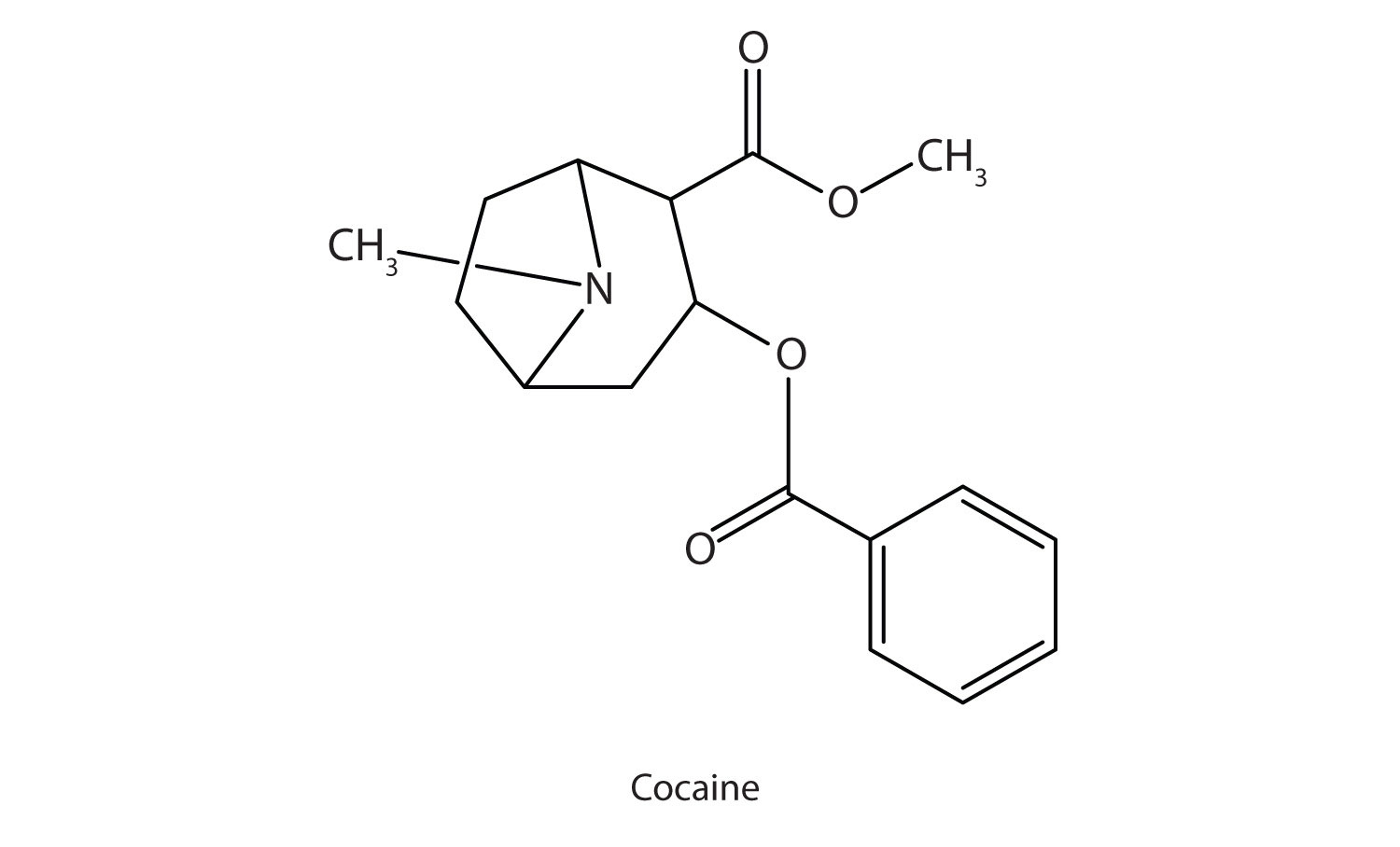
Cocaine is used as the salt cocaine hydrochloride and in the form of broken lumps of the free (unneutralized) base, which is called crack cocaine.
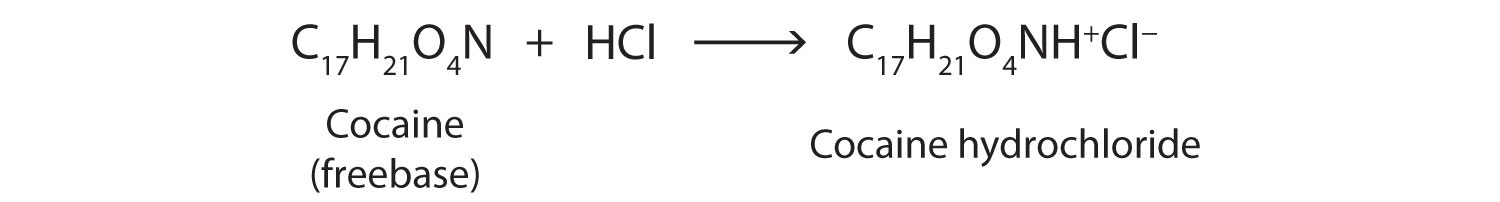
Because it is soluble in water, cocaine hydrochloride is readily absorbed through the watery mucous membranes of the nose when it is snorted. Crack cocaine is more volatile than cocaine hydrochloride. It vaporizes at the temperature of a burning cigarette. When smoked, cocaine reaches the brain in 15 s.
Explain the basicity of amines.
Contrast the physical properties of amines with those of alcohols and alkanes.
What is a heterocyclic compound?
Amines have a lone pair of electrons on the nitrogen atom and can thus act as proton acceptors (bases).
The solubilities of amines are similar to those of alcohols; the boiling points of primary and secondary amines are similar to those of alcohols; the boiling points of tertiary amines, which cannot engage in hydrogen bonding because they do not have a hydrogen atom on the nitrogen atom, are comparable to those of alkanes.
Heterocyclic compounds are ring compounds with atoms other than carbon atoms in the ring.
1. What salt is formed in each reaction? Write its condensed structural formula.
a. CH3NH2(aq) + HBr(aq) →
b. CH3NHCH3(aq) + HNO3(aq) →
2. What salt is formed in each reaction? Draw its structure.
a.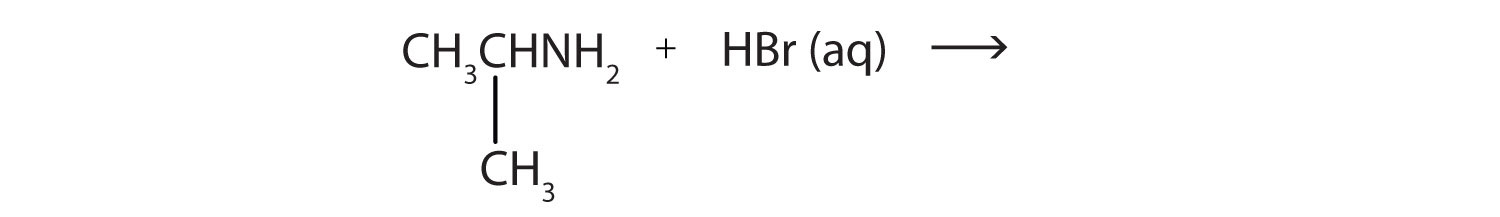
b.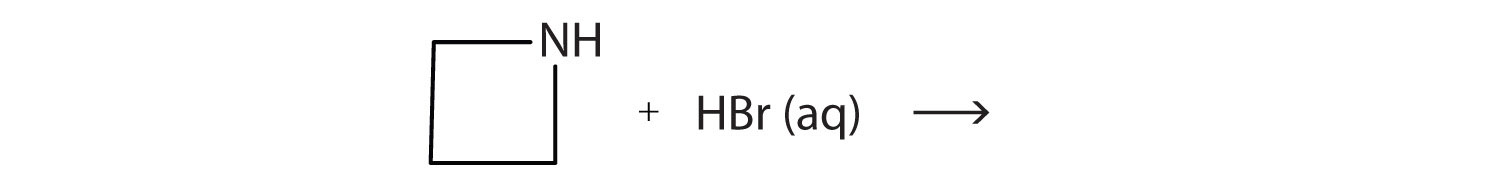
1.
a. CH3NH3+Br−(aq)
b. [CH3NH2CH3]+NO3−(aq)
The amide functional group has an nitrogen atom attached to a carbonyl carbon atom. If the two remaining bonds on the nitrogen atom are attached to hydrogen atoms, the compound is a simple amide. If one or both of the two remaining bonds on the atom are attached to alkyl or aryl groups, the compound is a substituted amide.
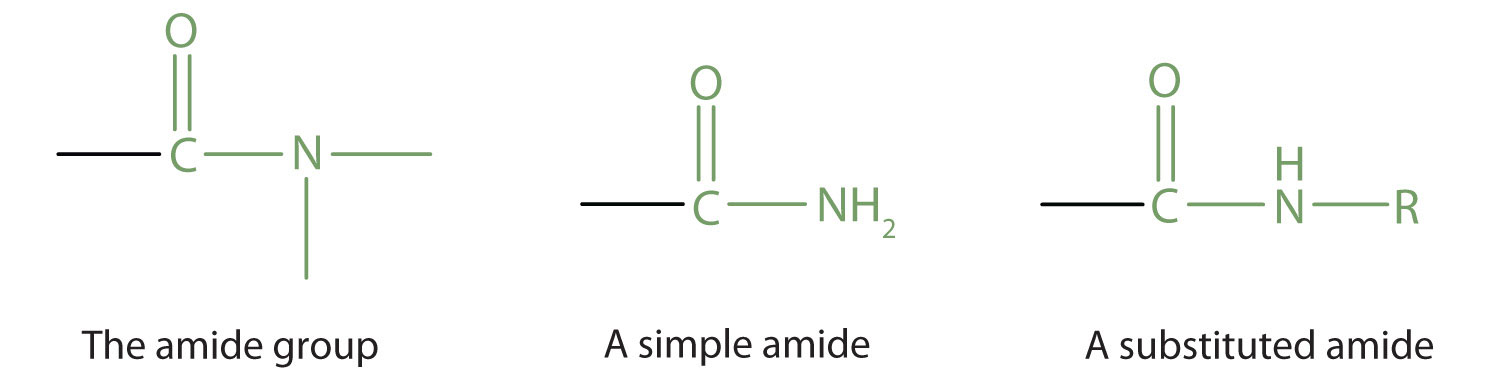
The carbonyl carbon-to-nitrogen bond is called an amide linkage. This bond is quite stable and is found in the repeating units of protein molecules, where it is called a peptide linkage. (For more about peptide linkages, see Chapter 8 "Amino Acids", Section 8.3 "Peptides".)
Simple amides are named as derivatives of carboxylic acids. The -ic ending of the common name or the -oic ending of the International Union of Pure and Applied Chemistry (IUPAC) name of the carboxylic acid is replaced with the suffix -amide.

Name each compound with the common name, the IUPAC name, or both.
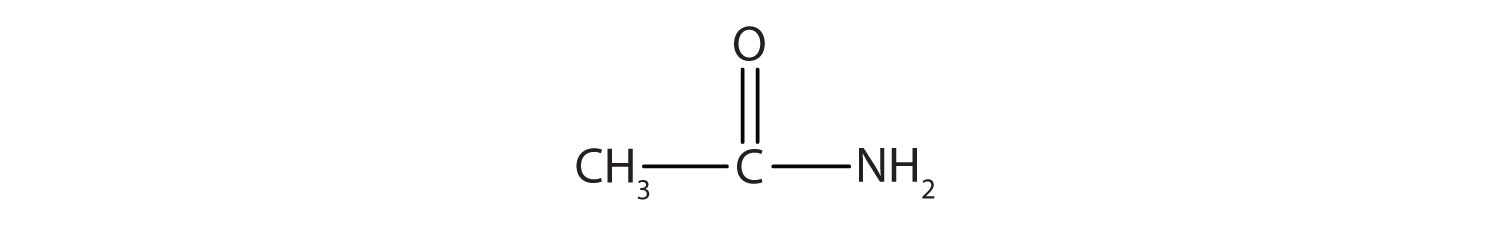
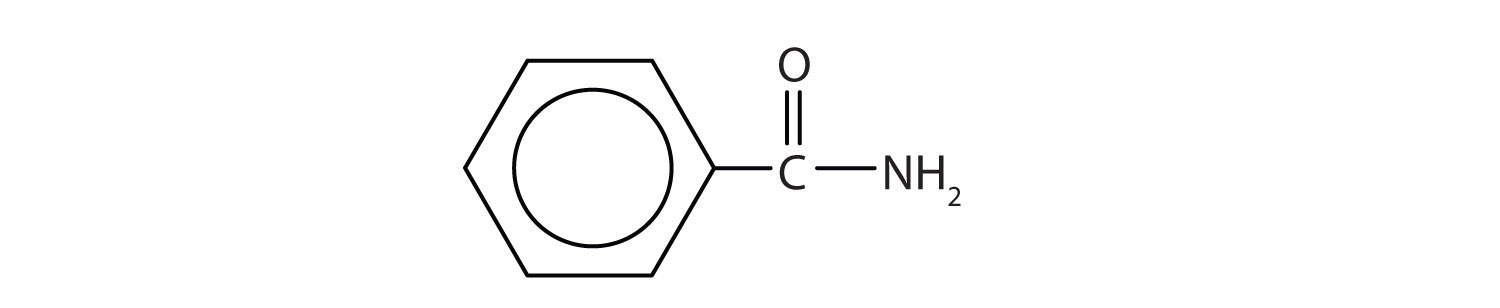
Solution
Name each compound with the common name, the IUPAC name, or both.
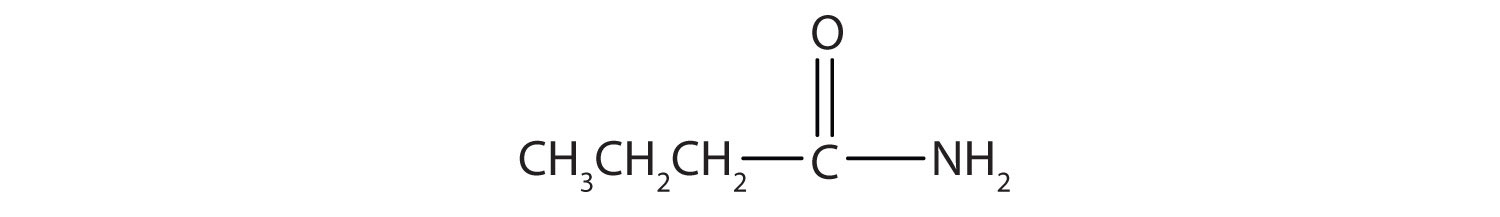
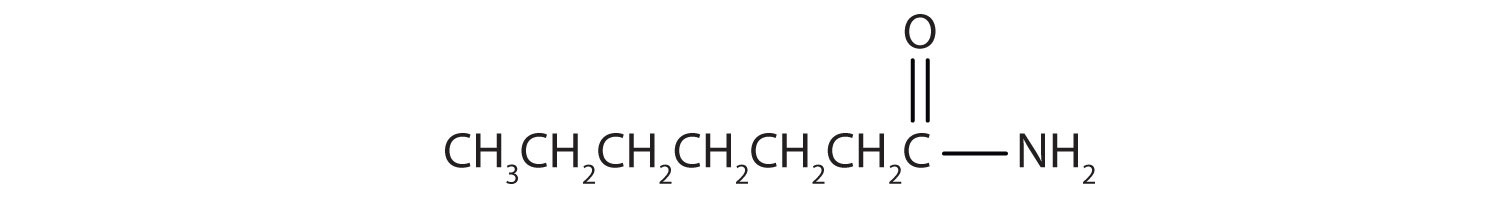
Name this compound with the common name and the IUPAC name.
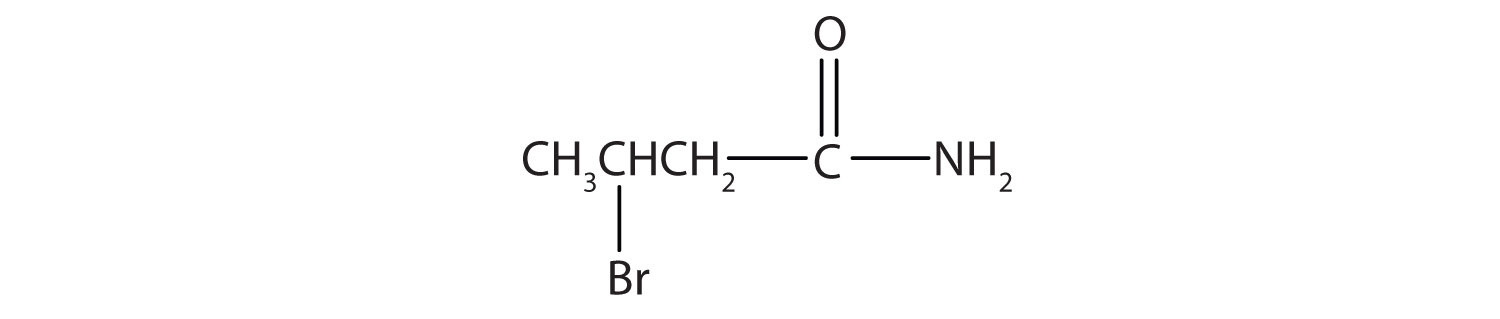
Draw a the structural formulae for pentanamide.
β-bromobutyramide (3-bromobutanamide)
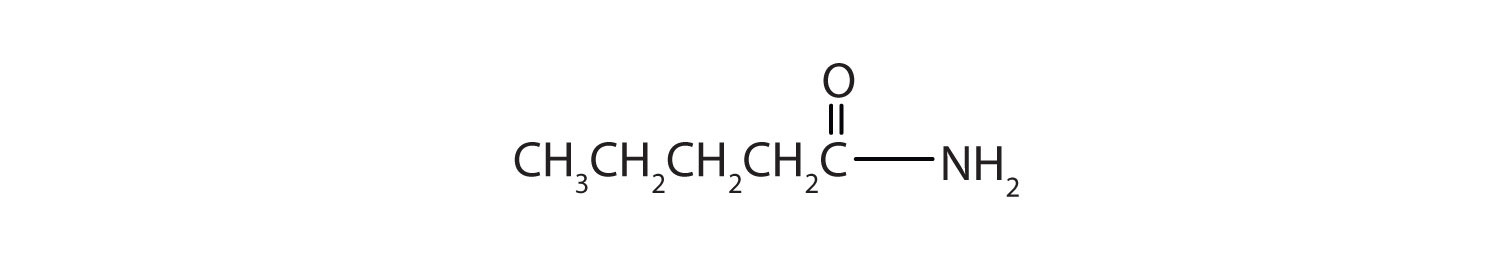
The functional group for an amide is as follows:
1. Draw the structure for each compound.
a. formamide
b. hexanamide
2. Draw the structure for each compound.
a. propionamide
b. butanamide
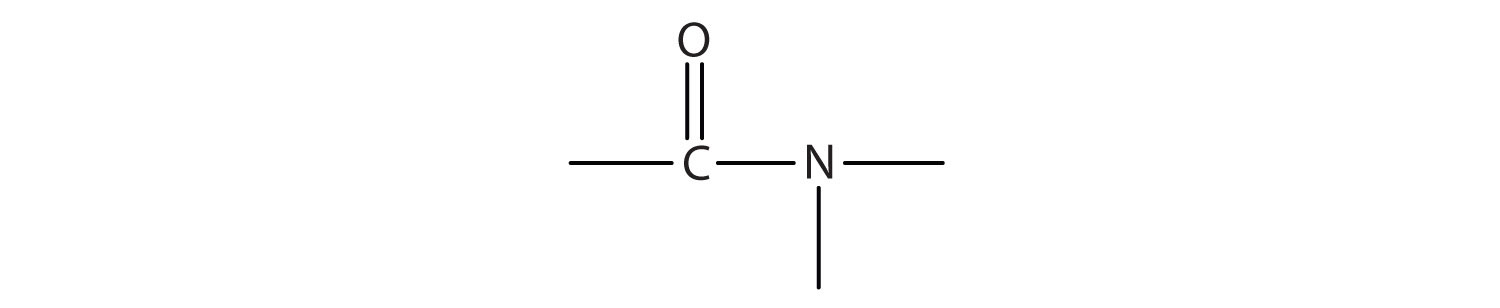
3. Name each compound with the common name, the IUPAC name, or both.
a. 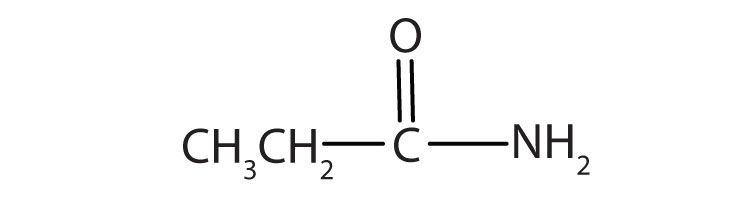
b.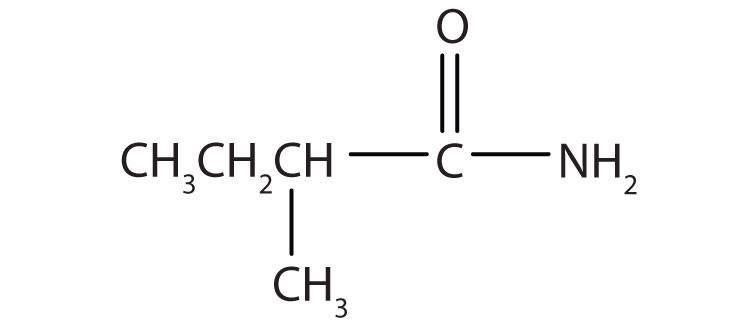
3. Name the compound.
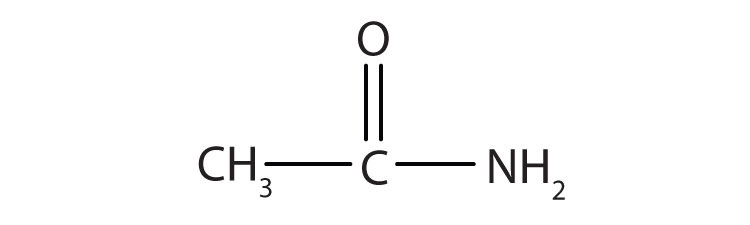
1.
a.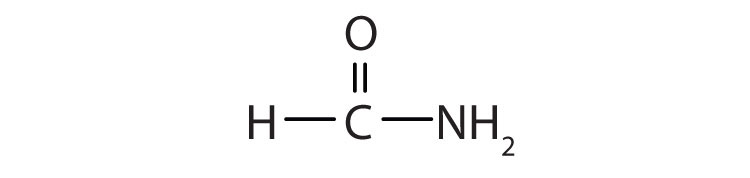
b.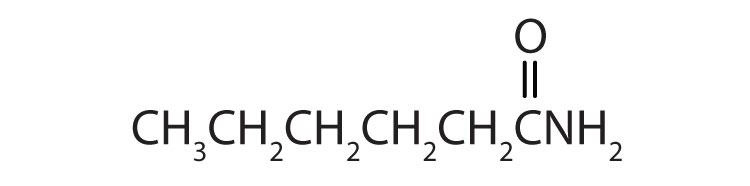
With the exception of formamide (HCONH2), which is a liquid, all simple amides are solids (Table 5.2 "Physical Constants of Some Unsubstituted Amides"). The lower members of the series are soluble in water, with borderline solubility occurring in those that have five or six carbon atoms. Like the esters, solutions of amides in water usually are neutral—neither acidic nor basic.
Table 5.2 Physical Constants of Some Unsubstituted Amides
| Condensed Structural Formula | Name | Melting Point (°C) | Boiling Point (°C) | Solubility in Water |
|---|---|---|---|---|
| HCONH2 | formamide | 2 | 193 | soluble |
| CH3CONH2 | acetamide | 82 | 222 | soluble |
| CH3CH2CONH2 | propionamide | 81 | 213 | soluble |
| CH3CH2CH2CONH2 | butyramide | 115 | 216 | soluble |
| C6H5CONH2 | benzamide | 132 | 290 | slightly soluble |
The amides generally have high boiling points and melting points. These characteristics and their solubility in water result from the polar nature of the amide group and hydrogen bonding (Figure 5.3 "Hydrogen Bonding in Amides"). (Similar hydrogen bonding plays a critical role in determining the structure and properties of proteins, deoxyribonucleic acid [DNA], ribonucleic acid [RNA], and other giant molecules so important to life processes. See Chapter 10 "Nucleic Acids and Protein Synthesis".)
Figure 5.3 Hydrogen Bonding in Amides
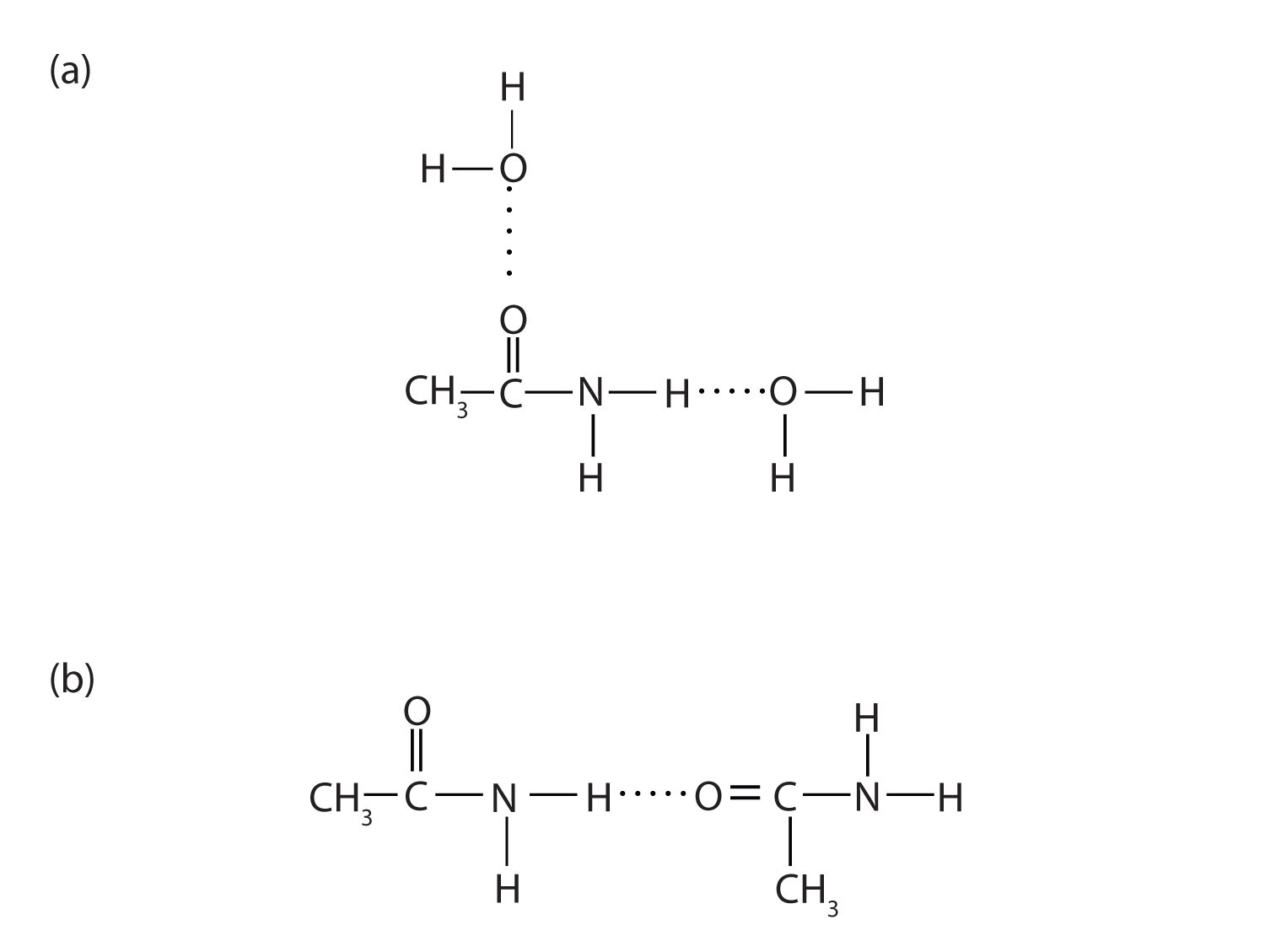
Amide molecules can engage in hydrogen bonding with water molecules (a). Those amides with a hydrogen atom on the nitrogen atom can also engage in hydrogen bonding (b). Both hydrogen bonding networks extend in all directions.
Which compound has the higher boiling point—pentanamide (CH3CH2CH2CH2CONH2) or propyl acetate (CH3COOCH2CH2CH3)? Explain.
Which compound is more soluble in water—propanamide (CH3CH2CONH2) or 1-pentene (CH2=CHCH2CH2CH3)? Explain.
pentanamide because the nitrogen-to-hydrogen (N–H) and the carbon-to-oxygen double (C=O) bonds can engage in hydrogen bonding; propyl acetate cannot engage in hydrogen bonding
propanamide because the N–H and C=O bonds can engage in hydrogen bonding with water; 1-pentene cannot engage in hydrogen bonding with water
1. Which compound has the higher boiling point—butyramide (CH3CH2CH2CONH2) or ethyl acetate (CH3COOCH2CH3)? Explain.
2. Which compound has the higher boiling point—butyramide or dimethylacetamide [CH3CON(CH3)2]? Explain.
3. Which compound is more soluble in water—acetamide (CH3CONH2) or 1-butene (CH2=CHCH2CH3)? Explain.
4. Which compound is more soluble in water—CH3CONHCH3 or 2-methylbutane [CH3CH(CH3)CH2CH3)]? Explain.
1. butyramide because the nitrogen-to-hydrogen (N–H) and the carbon-to-oxygen double (C=O) bonds can engage in hydrogen bonding; ethyl acetate cannot engage in hydrogen bonding
3. acetamide because the N–H and C=O bonds can engage in hydrogen bonding with water; 1-butene cannot engage in hydrogen bonding with water
The addition of ammonia (NH3) to a carboxylic acid forms an amide, but the reaction is very slow in the laboratory at room temperature. Water molecules are split out, and a bond is formed between the nitrogen atom and the carbonyl carbon atom.
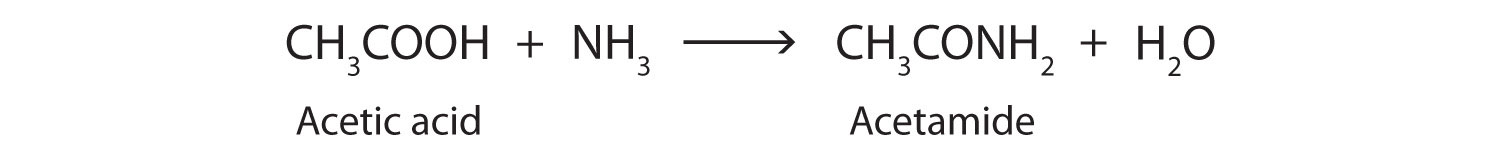
In living cells, amide formation is catalyzed by enzymes. Proteins are polyamides; they are formed by joining amino acids into long chains. In proteins, the amide functional group is called a peptide bond. (For more information about proteins, see Chapter 9 "Proteins and Enzymes", Section 9.1 "Proteins".)
Just as the reaction of a diol and a diacid forms a polyester (see Chapter 4 "Carboxylic Acids, Esters" Section 4.8 "Preparation of Esters"), the reaction of a diacid and a diamine yields a polyamide. The two difunctional monomers often employed are adipic acid and 1,6-hexanediamine. The monomers condense by splitting out water to form a new product, which is still difunctional and thus can react further to yield a polyamide polymer.
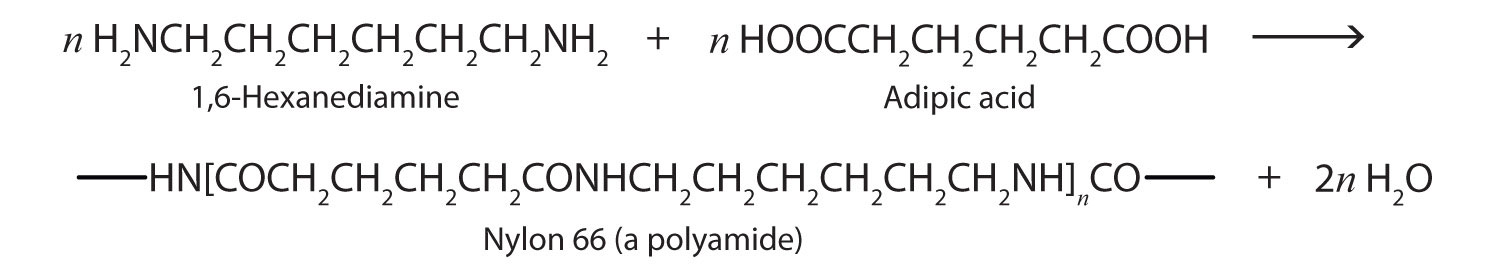
Some polyamides are known as nylons. Nylons are among the most widely used synthetic fibers—for example, they are used in ropes, sails, carpets, clothing, tires, brushes, and parachutes. They also can be molded into blocks for use in electrical equipment, gears, bearings, and valves.
Write the condensed structural formulas and give names of the two compounds from which butanamide (CH3CH2CH2CONH2) is formed.
Write the condensed structural formulas and names of the two compounds from which CH3CH2CH2CH2CH2CONHCH2CH2CH3 is formed.
CH3CH2CH2COOH (butanoic acid) and NH3 (ammonia)
CH3CH2CH2CH2CH2COOH (hexanoic acid) and CH3CH2CH2NH2 (propylamine)
1. Write the condensed structural formulas and names of the two compounds from which pentanamide (CH3CH2CH2CH2CONH2) is formed.
2. Write the condensed structural formulas and names of the two compounds from which CH3CONHCH3 is formed.
1. CH3CH2CH2CH2COOH (pentanoic acid) and NH3 (ammonia)
Generally, amides resist hydrolysis in plain water, even after prolonged heating. In the presence of added acid or base, however, hydrolysis proceeds at a moderate rate. In living cells, amide hydrolysis is catalyzed by enzymes. Amide hydrolysis is illustrated in the following example:
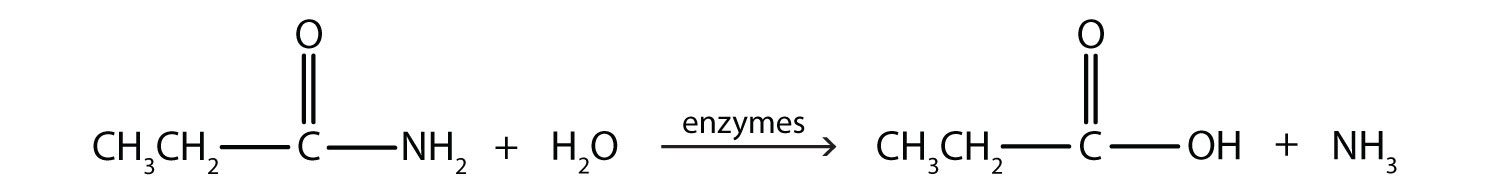
Hydrolysis of an amide in acid solution actually gives a carboxylic acid and the i>salt of ammonia or an amine (the ammonia or amine initially formed is neutralized by the acid). Basic hydrolysis gives a salt of the carboxylic acid and ammonia or an amine.
Write the equation for the hydrolysis of each compound.
Solution
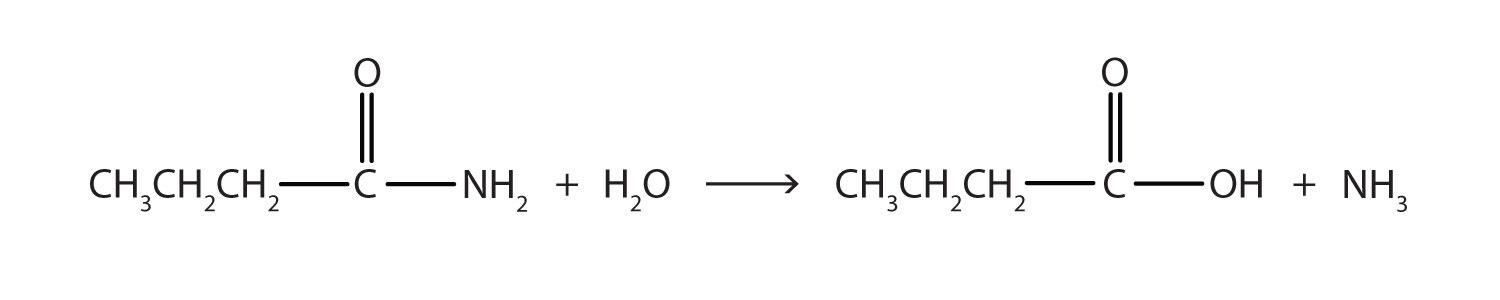
The hydrolysis of a simple amide produces an organic acid and ammonia. Butyramide thus yields butyric acid and ammonia.
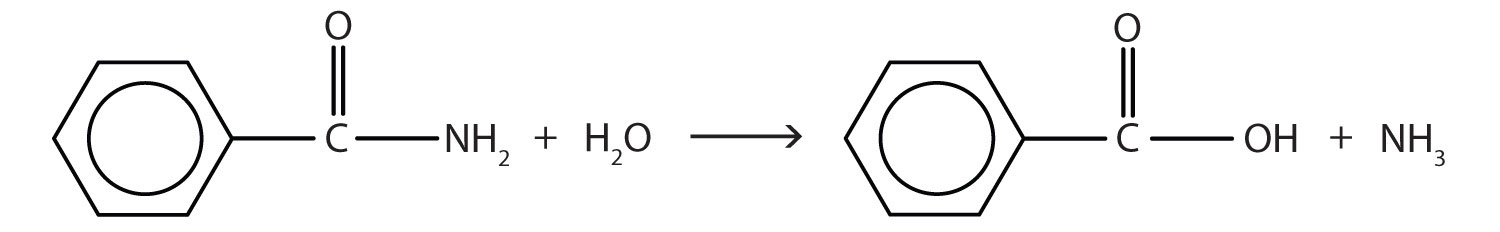
The hydrolysis of an amide produces an organic acid and ammonia. Benzamide thus yields benzoic acid and ammonia.
Write the equation for the hydrolysis of each compound.
propionamide (propanamide)
hexanamide
Athletic training is an allied health-care profession recognized by the American Medical Association. The athletic trainer’s role is to recognize, evaluate, and provide immediate care for athletic injuries; prevent athletic injuries by taping, bandaging, and bracing vulnerable body parts; make referrals to medical doctors when necessary; and rehabilitate injured athletes. Athletic trainers work in high schools, colleges, and other organizations where athletics programs are found. Athletic trainers usually have a degree from an accredited athletic training program whose curriculum includes such basic science courses as biology, chemistry, and physics. These studies provide the necessary background for more applied courses, such as anatomy and physiology, exercise physiology, kinesiology, and nutrition. Knowledge of chemistry is necessary for understanding pharmacological and medical terminology. For example, athletic trainers must understand the action of numerous drugs, many of which are esters, amines, or amides like those mentioned in this chapter.
Athletic trainers may have administrative duties, such as the responsibility for ordering supplies. They also need to be able to evaluate nutritional supplements because providing the wrong one can get an athlete banned from competition and may bring sanctions against a school. In short, the athletic trainer is responsible for the overall health and well-being of the athletes in his or her charge.
What are the products of the hydrolysis of an amide?
When the amide CH3CH2CH2CH2CONH2 is hydrolyzed in an NaOH solution, the products are CH3CH2CH2CH2COO−Na+ and NH3. What products are obtained when CH3CH2CH2CH2CONH2 is hydrolyzed in an hydrochloric acid solution?
a carboxylic acid and ammonia or an amine
CH3CH2CH2CH2COOH and NH4Cl
1. Complete each equation.
a.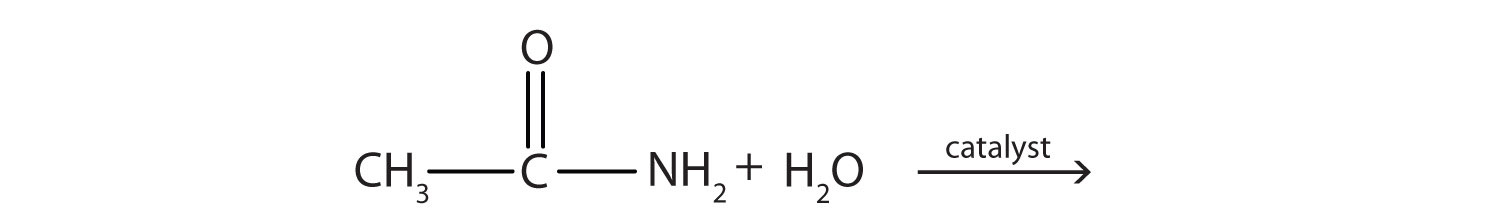
b.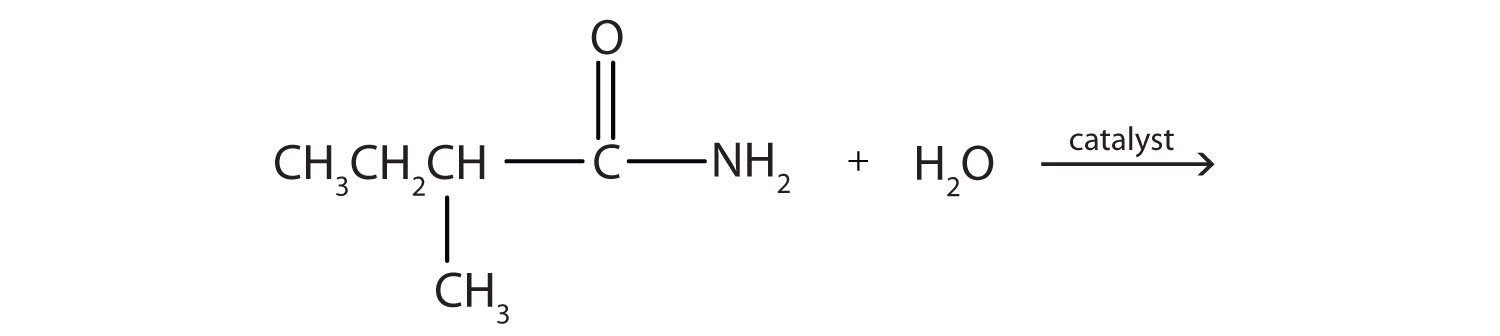
2. Complete each equation.
a.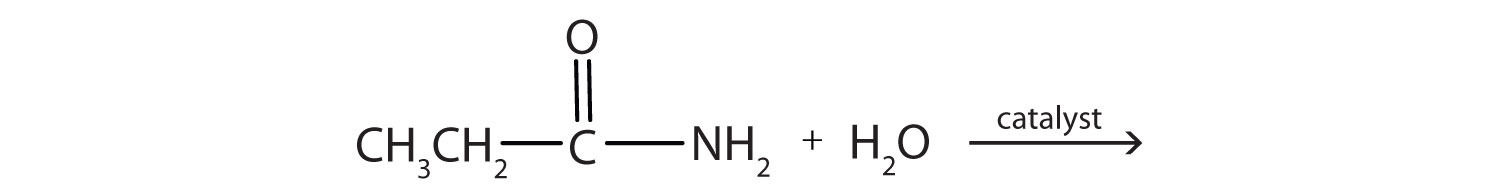
b.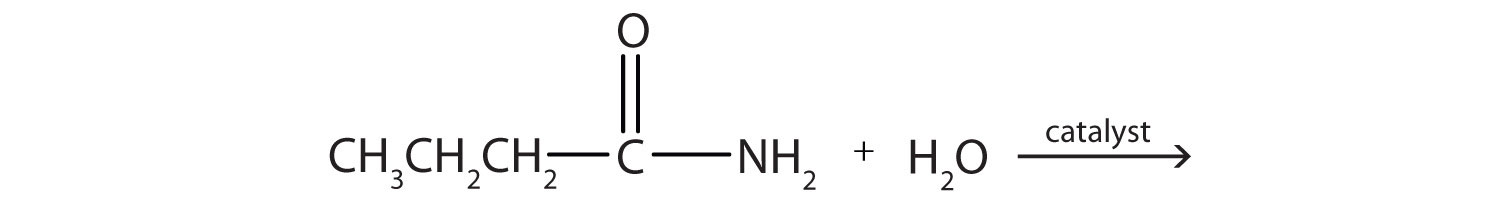
1.
a. CH3COOH + NH3
b.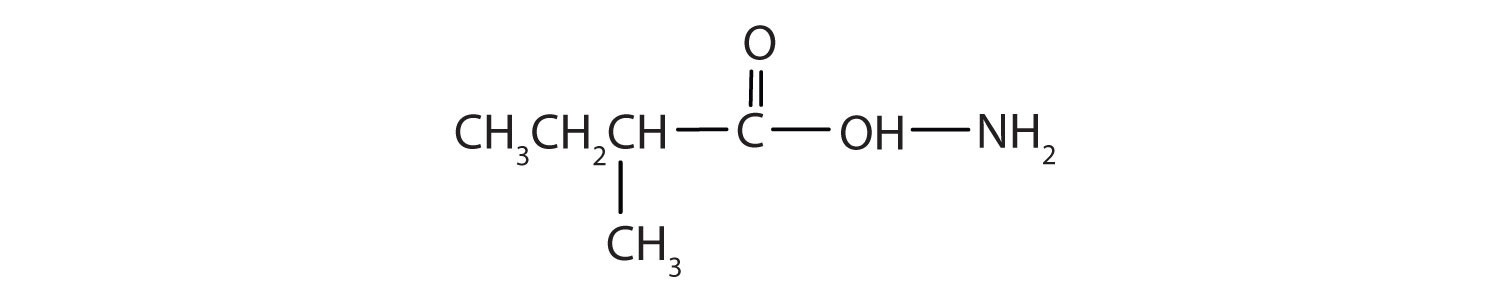
To ensure that you understand the material in this chapter, you should review the meanings of the following bold terms in the summary and ask yourself how they relate to the topics in the chapter.
A carboxylic acid (RCOOH) contains the functional group COOH, called the carboxyl group, which has an OH group attached to a carbonyl carbon atom. An ester (RCOOR′) has an OR′ group attached to a carbonyl carbon atom. An amine is derived from ammonia (NH3), with one, two, or all three of the hydrogen atoms of NH3 replaced by an alkyl (or an aryl) group. The amide functional group has a carbonyl group joined to a nitrogen atom from NH3 or an amine.
There are many familiar carboxylic acids. The R group may be a hydrogen atom (as in formic acid, HCOOH), an alkyl group (as in acetic acid, CH2COOH), or an aryl group (as in benzoic acid, C6H5COOH). The location of substituents along the carbon chain is indicated by a Greek letter (for common names) or a number (for names from the International Union of Pure and Applied Chemistry).
A carboxylic acid is formed by the oxidation of an aldehyde with the same number of carbon atoms. Because aldehydes are formed from primary alcohols, these alcohols are also a starting material for carboxylic acids.
Carboxylic acids have strong, often disagreeable, odors. They are highly polar molecules and readily engage in hydrogen bonding, so they have relatively high boiling points.
Carboxylic acids are weak acids. They react with bases to form salts and with carbonates and bicarbonates to form carbon dioxide gas and the salt of the acid.
Esters are pleasant-smelling compounds that are responsible for the fragrances of flowers and fruits. They have lower boiling points than comparable carboxylic acids because, even though ester molecules are somewhat polar, they cannot engage in hydrogen bonding. However, with water, esters can engage in hydrogen bonding; consequently, the low molar mass esters are soluble in water. Esters can be synthesized by esterification, in which a carboxylic acid and an alcohol are combined under acidic conditions. Esters are neutral compounds that undergo hydrolysis, a reaction with water. Under acidic conditions, hydrolysis is essentially the reverse of esterification. When carried out under basic conditions, the process is called saponification.
Inorganic acids also react with alcohols to form esters. Some of the most important esters in biochemistry are those formed from phosphoric acid.
Amines are nitrogen-containing organic molecules derived from ammonia (NH3). A primary (1°) amine (RNH2) has one organic group bonded to the nitrogen atom, a secondary (2°) amine (R2NH) has two organic groups bonded to the nitrogen atom, and a tertiary (3°) amine (R3N) has three organic groups bonded to the nitrogen atom. Amines are basic compounds that react with strong acids to produce ammonium (NH4+) salts. A cyclic compound in which the ring contains one or more noncarbon atoms is called a heterocyclic compound. There are many heterocyclic amines, including many physiologically important ones. Alkaloids are heterocyclic amines found in many plants. Caffeine, nicotine, and cocaine are familiar alkaloids.
Organic compounds containing a carbonyl group bonded to a nitrogen atom are amides, and the carbon-to-nitrogen bond is an amide linkage (or a peptide linkage). Most amides are colorless and odorless, and the lighter ones are soluble in water. Because they are polar molecules, amides have comparatively high boiling points and melting points. Amides are synthesized from carboxylic acids and NH3 or amines. Amides are neutral compounds. They resist hydrolysis in water, but acids, bases, and enzymes catalyze the reaction.
1. Of the families of organic compounds discussed in this chapter, which are known for their typically unpleasant odors? Which for their characteristically pleasant aromas?
2. What is esterification of a carboxylic acid? How does it differ from neutralization?
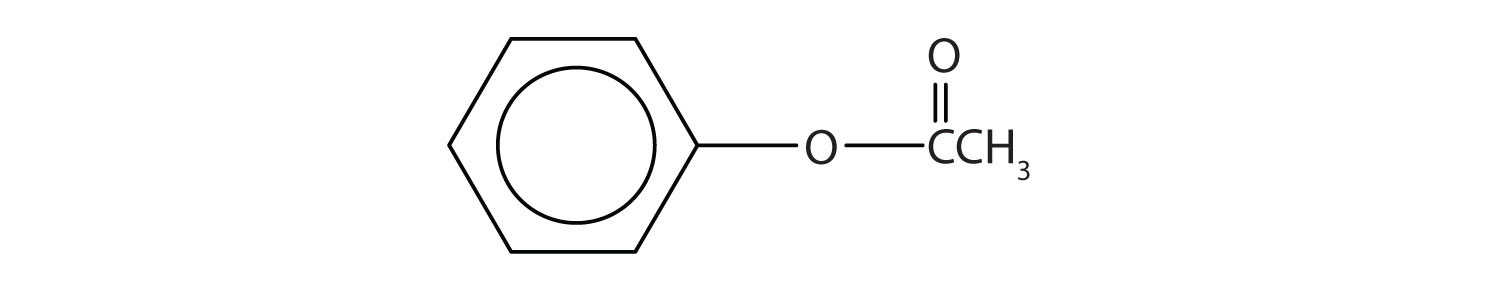
3. Like alcohols, phenols form esters with carboxylic acids. The hydrocarbon group from phenol is called phenyl. Draw the structure of phenyl acetate.
4. Describe the hydrogen bonding in carboxylic acids, both acid-acid and acid-water. How does this influence their physical properties?
5. Which compound is more soluble in water—benzoic acid or sodium benzoate? Explain.
6. Dicarboxylic acids have two carboxyl groups and are named with the ending -dioic acid. Give the equation for the reaction of 1,5-pentanedioic acid (HOOCCH2CH2CH2COOH; common name, glutaric acid) with each of the following:
s. 1 mol of NaOH
b. 2 mol of NaOH
7. Without consulting tables, arrange the following compounds in order of increasing boiling point: butyl alcohol, methyl acetate, pentane, and propionic acid.
8. From which alcohol might each acid be prepared via oxidation with acidic dichromate?
a. CH3CH2COOH
b. HCOOH
c. HOOCH2COOH
d. (CH3)2CHCH2COOH
9. The distinctive aroma and flavor of oranges are due in part to octyl acetate, an ester formed from 1-octanol (octyl alcohol) and acetic acid. Write the condensed structural formula for octyl acetate.
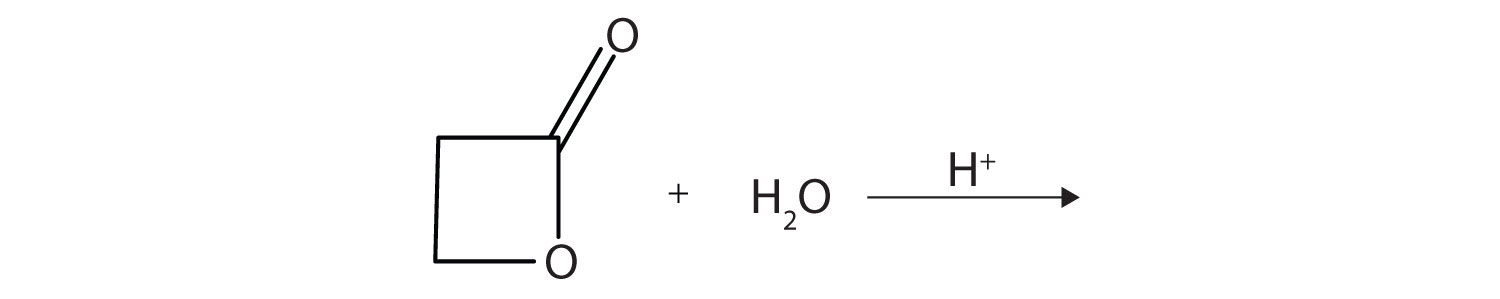
10. A lactone is a cyclic ester. What product is formed in following reaction?
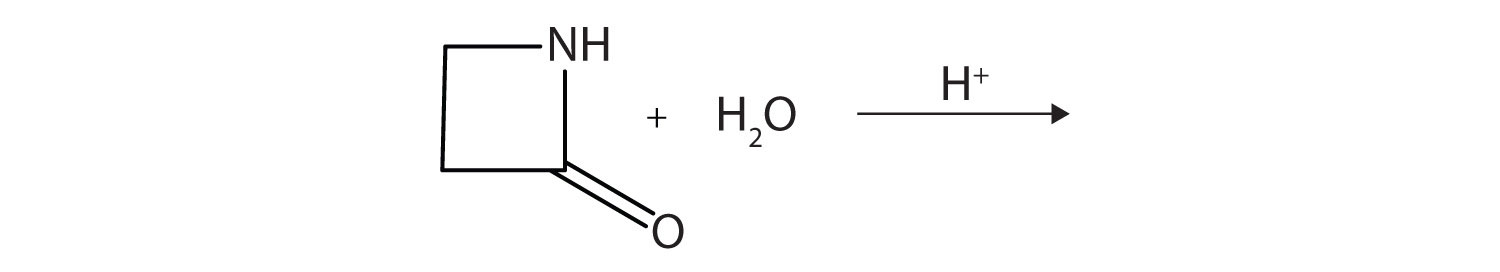
11. A lactam is a cyclic amide. What product is formed in the following reaction?
12. Draw the structures for the eight isomeric amines that have the molecular formula C4H11N. Give each a common name and classify it as primary, secondary, or tertiary.
13. Draw the structures for the five isomeric amines that have the molecular formula C7H9N and contain a benzene ring. Classify each compound as primary, secondary, or tertiary.
14. Cocaine is usually used in the form of the salt cocaine hydrochloride and sniffed up the nose. Some prefer to ingest their cocaine by smoking it (mixed with tobacco, for example). Before smoking, the cocaine hydrochloride must be converted back to the free base (that is, to the molecular form). Explain the choice of dosage form for each route of administration.
15. Draw the structures all the isomeric amides that have the molecular formula C4H9NO.
16. An ester with the molecular formula C6H12O2 was hydrolyzed in aqueous acid to yield an acid Y and an alcohol Z. Oxidation of the alcohol with potassium dichromate (K2Cr2O7) gave the identical acid Y. What is the condensed structural formula of the ester?
17. The neutralization of 125 mL of a 0.400 M NaOH solution requires 5.10 g of a monocarboxylic acid. Draw all the possible structures for the acid.
18. If 3.00 g of acetic acid reacts with excess methanol, how many grams of methyl acetate are formed?
19. How many milliliters of a 0.100 M barium hydroxide solution are required to neutralize 0.500 g of dichloroacetic acid?
1. unpleasant: carboxylic acids; pleasant: esters
3.
5. sodium benzoate because it is ionic and forms ion-dipole forces with water; benzoic acid can engage in hydrogen bonding only with water
7. pentane < methyl acetate < butyl alcohol < propionic acid
9. CH3COOCH2CH2CH2CH2CH2CH2CH2CH3
11. H3N+CH2CH2COOH
13.
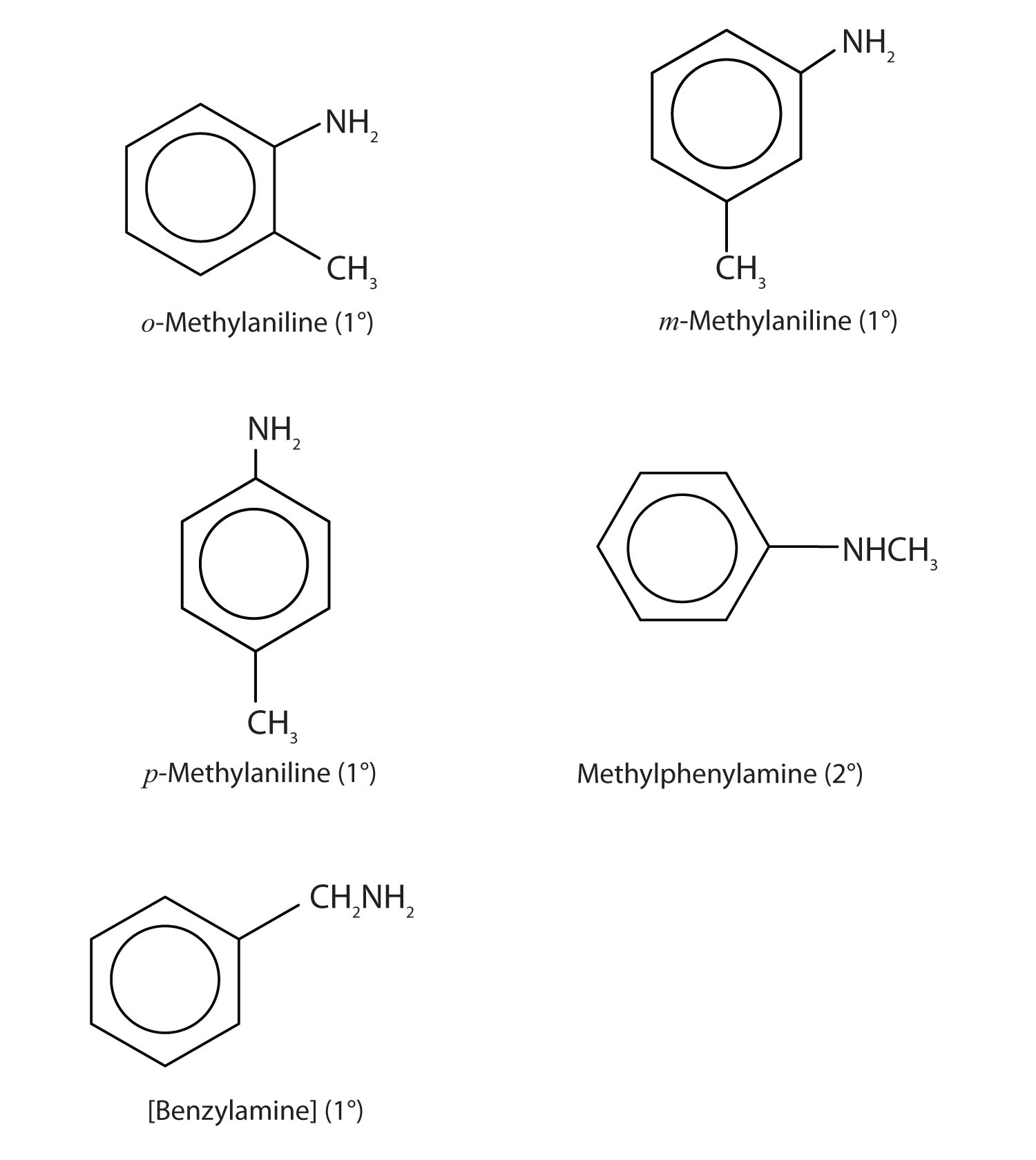
15.
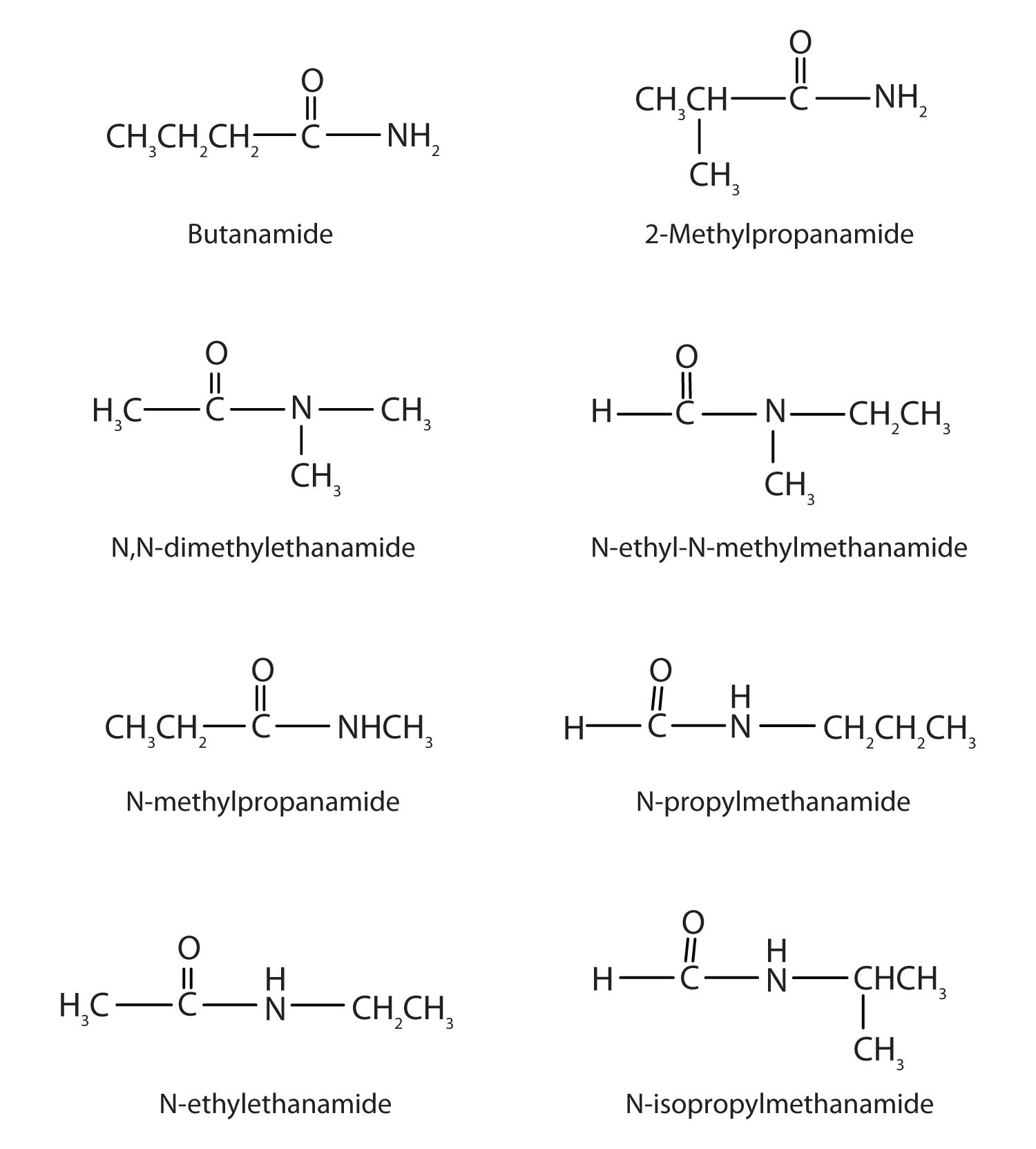
17.
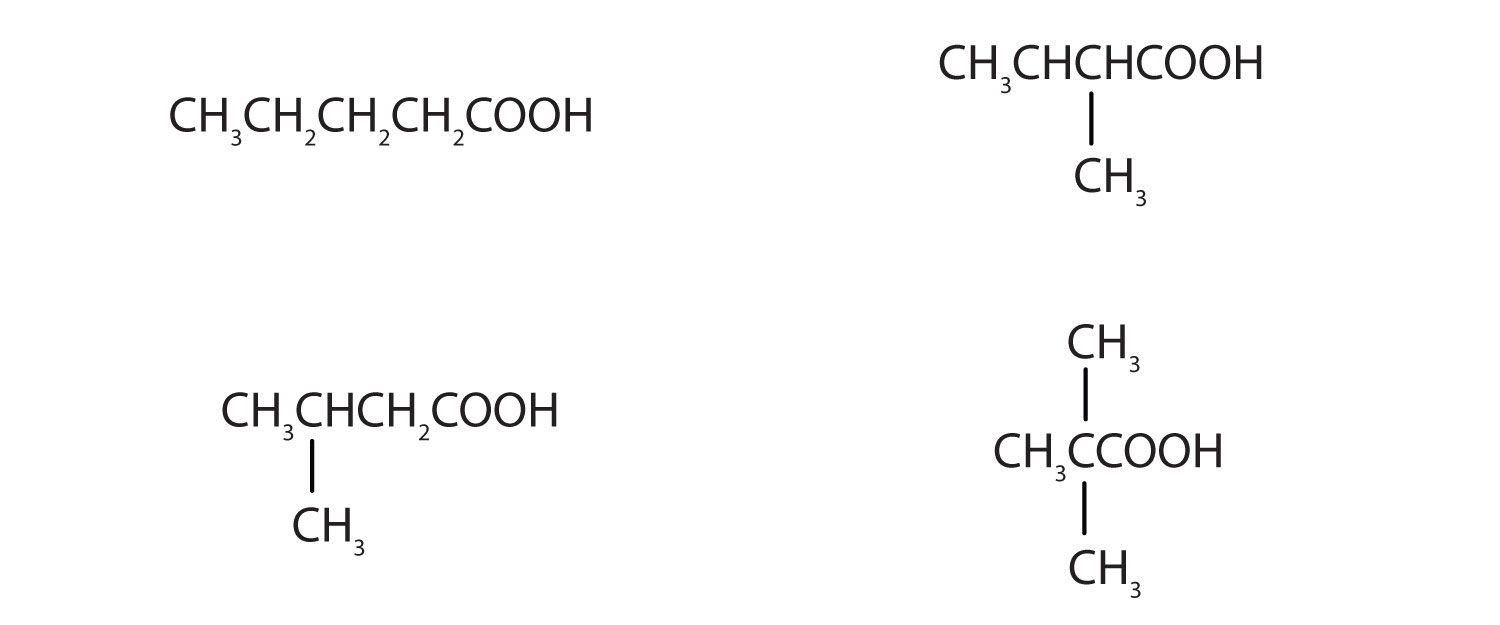
19. 20.0 mL
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
