Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball, John W. Hill, and Rhonda J. Scott
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
One of the more familiar chemical compounds on Earth is ethyl alcohol (ethanol). As the intoxicant in alcoholic beverages, ethanol is often simply called alcohol. If ethanol is diluted, as it is in wine, beer, or mixed drinks with about 1 oz of liquor, and if it is consumed in small quantities, it is relatively safe. In excess—four or more drinks in a few hours—it causes intoxication, which is characterized by a loss of coordination, nausea and vomiting, and memory blackouts.
Excessive ingestion of ethanol over a long period of time leads to cirrhosis of the liver, alteration of brain cell function, nerve damage, and strong physiological addiction. Alcoholism—an addiction to ethanol—is the most serious drug problem in the United States. Heavy drinking shortens a person’s life span by contributing to diseases of the liver, the cardiovascular system, and virtually every other organ of the body.
In small quantities—one or two drinks a day—ethanol might promote health. In addition to the possible benefits of modest amounts of ethanol, a chemical in red wines, resveratrol, is thought to lower the risk of heart disease. Resveratrol, found in red grapes, is an antioxidant. It inhibits the oxidation of cholesterol and subsequent clogging of the arteries. One need not drink wine to get the benefits of resveratrol, however. It can be obtained by eating the grapes or drinking red grape juice.
Ethanol and resveratrol, a phenol, are representatives of two of the families of oxygen-containing compounds that we consider in this chapter. Two other classes, aldehydes and ketones, are formed by the oxidation of alcohols. Ethers, another class, are made by the dehydration of alcohols.
In Chapter 1"Organic Chemistry Review / Hydrocarbons", we considered several kinds of hydrocarbons. Now we examine some of the many organic compounds that contain functional groups. If you understand the behavior of a particular functional group, you will know a great deal about the general properties of that class of compounds. In this chapter, Chapter 3 "Aldehydes, Ketones", and Chapter 4 "Carboxylic Acids, Esters", we make a brief yet systematic study of some of organic compound families. Each family is based on a common, simple functional group that contains an oxygen atom or a nitrogen atom. Some common functional groups are listed in Table 2.1 "Selected Organic Functional Groups".
Table 2.1 Selected Organic Functional Groups
| Name of Family | General Formula | Functional Group | Suffix* |
|---|---|---|---|
| alkane | RH | none | -ane |
| alkene | R2C=CR2 |
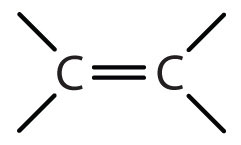 |
-ene |
| alkyne | RC≡CR | –C≡C– | -yne |
| alcohol | ROH | –OH | -ol |
| thiol | RSH | –SH | -thiol |
| ether | ROR | –O– | ether |
| aldehyde |
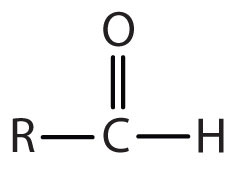 |
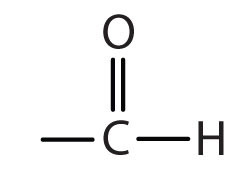 |
-al |
| ketone |
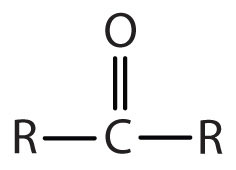 |
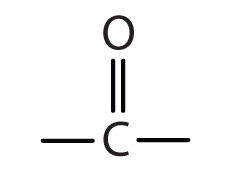 |
-one |
| carboxylic acid |
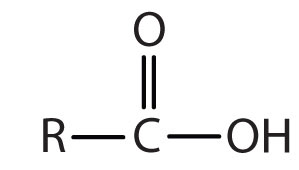 |
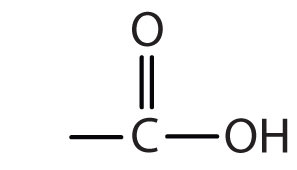 |
-oic acid |
*Ethers do not have a suffix in their common name; all ethers end with the word ether.
What is the functional group of an alkene? An alkyne?
Does CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3 have a functional group? Explain.
carbon-to-carbon double bond; carbon-to-carbon triple bond
No; it has nothing but carbon and hydrogen atoms and all single bonds.
What is the functional group of 1-butanol (CH3CH2CH2CH2OH)?
What is the functional group of butyl bromide, CH3CH2CH2CH2Br?
OH
An alcohol is an organic compound with a hydroxyl (OH) functional group on an aliphatic carbon atom. Because OH is the functional group of all alcohols, we often represent alcohols by the general formula ROH, where R is an alkyl group. (For more information about alkyl groups, see Chapter 1 "Organic Chemistry Review / Hydrocarbons", Section 1.5 "IUPAC Nomenclature". Table 1.4 "Common Alkyl Groups" presents some common alkyl groups.)
Alcohols are common in nature. Most people are familiar with ethyl alcohol (ethanol), the active ingredient in alcoholic beverages, but this compound is only one of a family of organic compounds known as alcohols. The family also includes such familiar substances as cholesterol and the carbohydrates.
Methanol (CH3OH) and ethanol (CH3CH2OH) are the first two members of the homologous series of alcohols.
Alcohols with one to four carbon atoms are frequently called by common names, in which the name of the alkyl group is followed by the word alcohol:
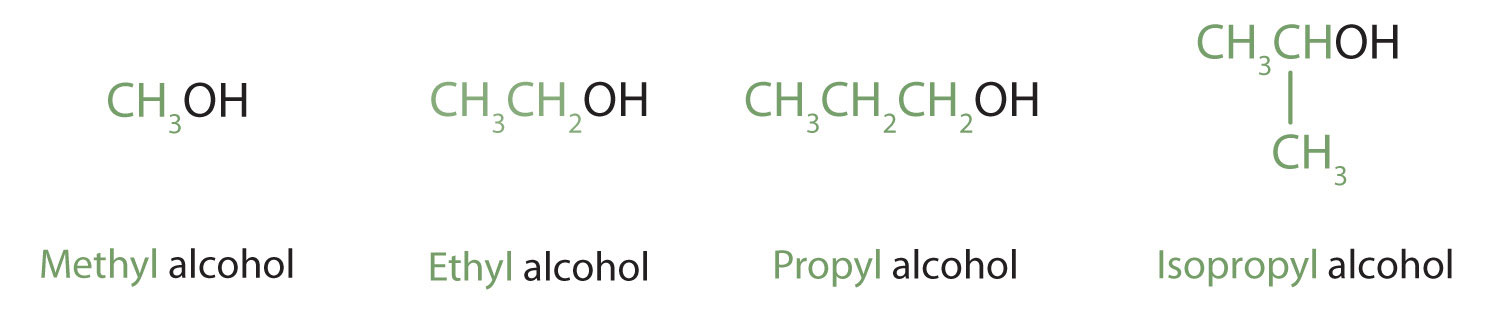
According to the International Union of Pure and Applied Chemistry (IUPAC), alcohols are named by changing the ending of the parent alkane name (Chapter 1 "Organic Chemistry Review / Hydrocarbons", Section 1.5 "IUPAC Nomenclature") to -ol. Here are some basic IUPAC rules for naming alcohols:
Figure 2.1 "IUPAC Rules for Alcohols" shows some examples of the application of these rules.
Figure 2.1 IUPAC Rules for Alcohols
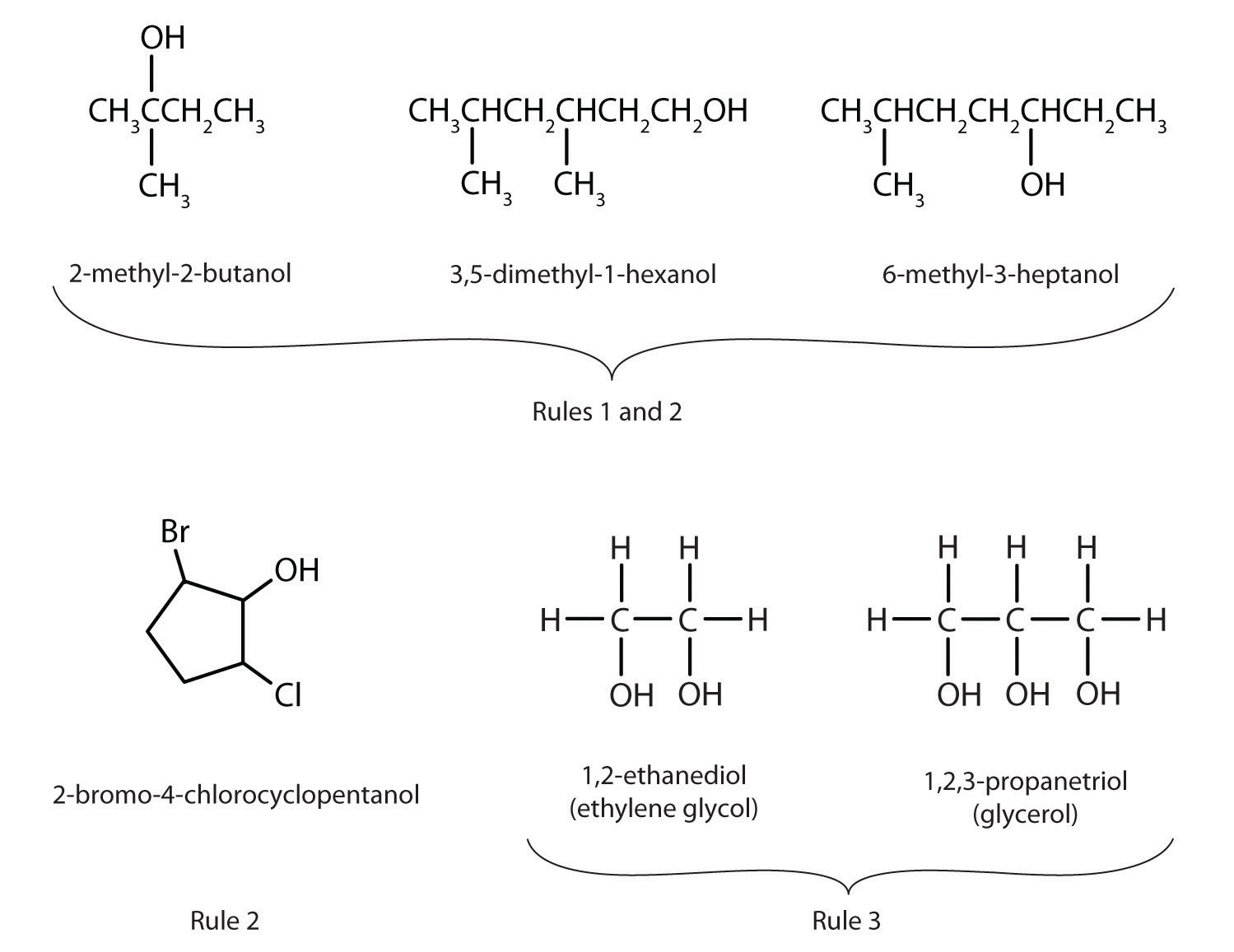
The names and structures of some alcohols demonstrate the use of IUPAC rules.
Give the IUPAC name for each compound.
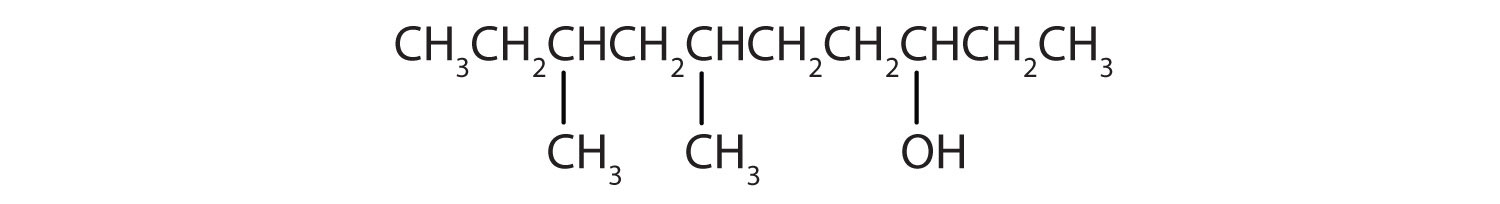
Ten carbon atoms in the LCC makes the compound a derivative of decane (rule 1), and the OH on the third carbon atom makes it a 3-decanol (rule 2).
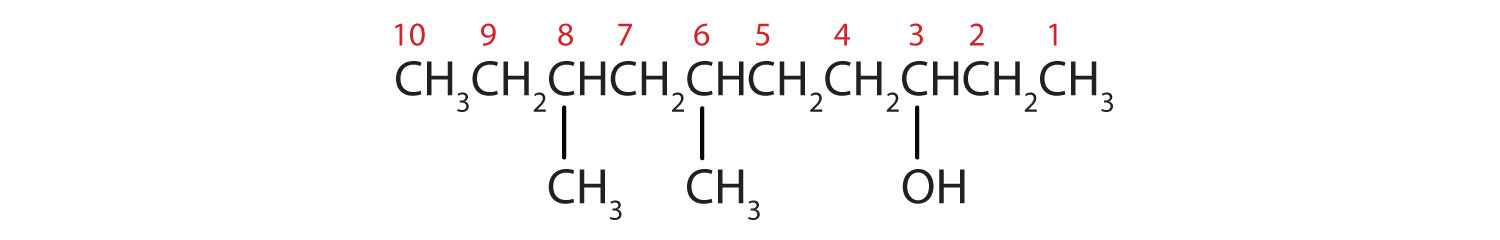
The carbon atoms are numbered from the end closest to the OH group. That fixes the two methyl (CH3) groups at the sixth and eighth positions. The name is 6,8-dimethyl-3-decanol (not 3,5-dimethyl-8-decanol).
Five carbon atoms in the LCC make the compound a derivative of pentane. Two OH groups on the first and fifth carbon atoms make the compound a diol and give the name 1,5-pentanediol (rule 3).
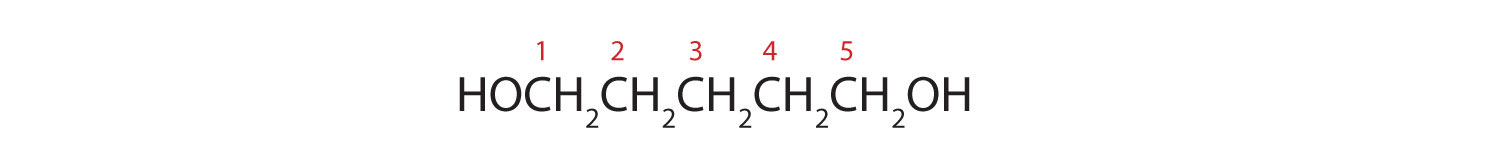
Give the IUPAC name for each compound.
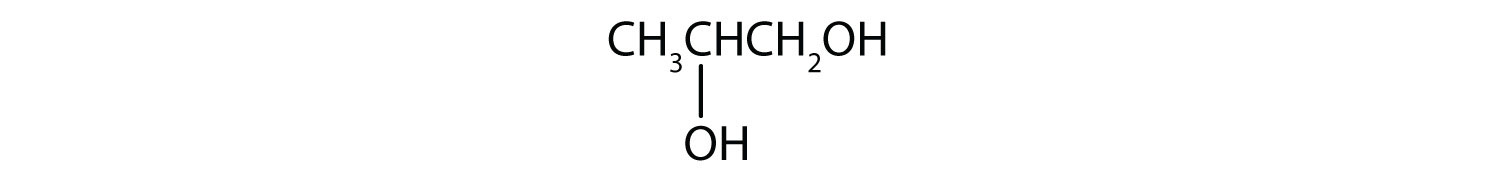
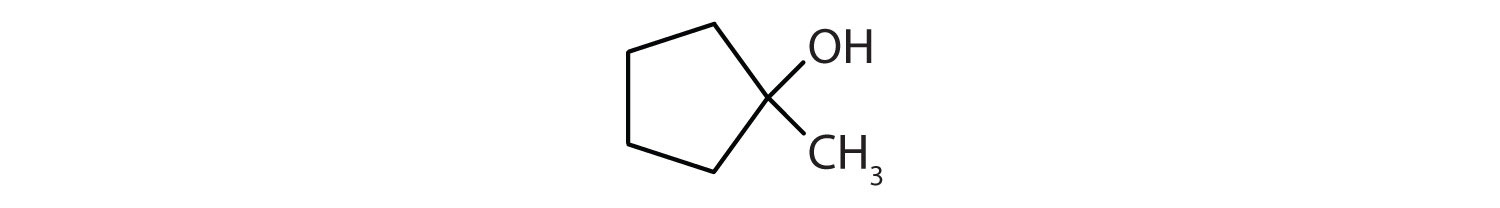
Draw the structure for each compound.
The ending -ol indicates an alcohol (the OH functional group), and the hex- stem tells us that there are six carbon atoms in the LCC. We start by drawing a chain of six carbon atoms: –C–C–C–C–C–C–.
The 2 indicates that the OH group is attached to the second carbon atom.
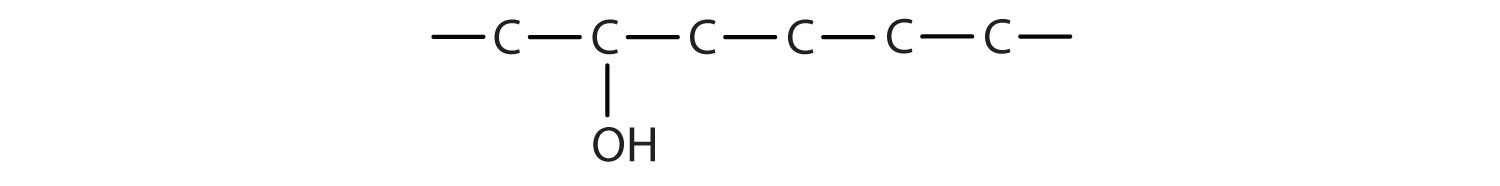
Finally, we add enough hydrogen atoms to give each carbon atom four bonds.
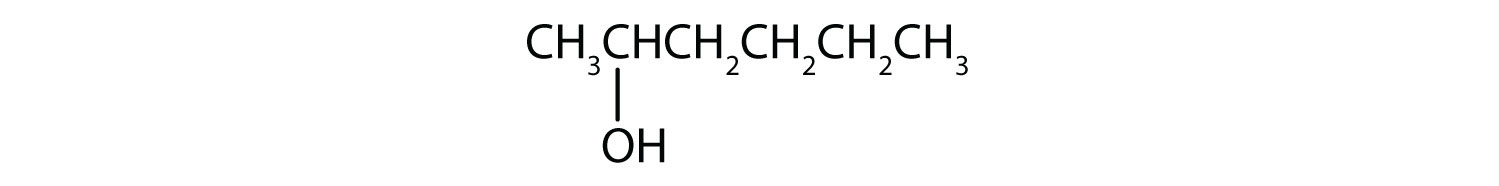
The ending -ol indicates an OH functional group, and the pent- stem tells us that there are five carbon atoms in the LCC. We start by drawing a chain of five carbon atoms:
–C–C–C–C–C–The numbers indicate that there is a methyl (CH3) group on the third carbon atom and an OH group on the second carbon atom.
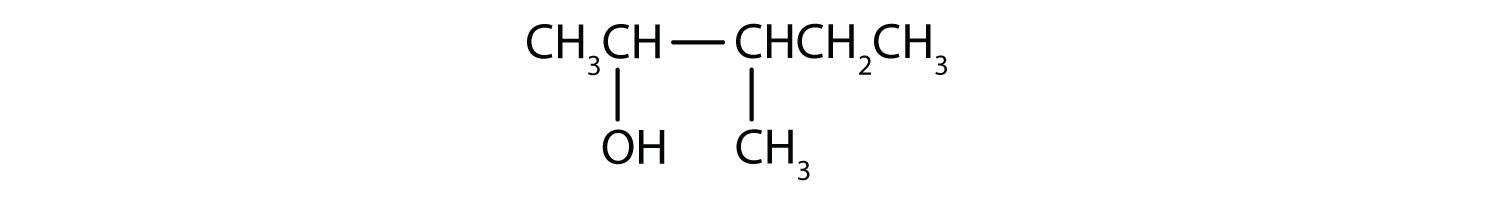
Draw the structure for each compound.
3-heptanol
2-methyl-3-hexanol
Some of the properties of alcohols depend on the number of carbon atoms attached to the specific carbon atom that is attached to the OH group. Alcohols can be grouped into three classes on this basis.
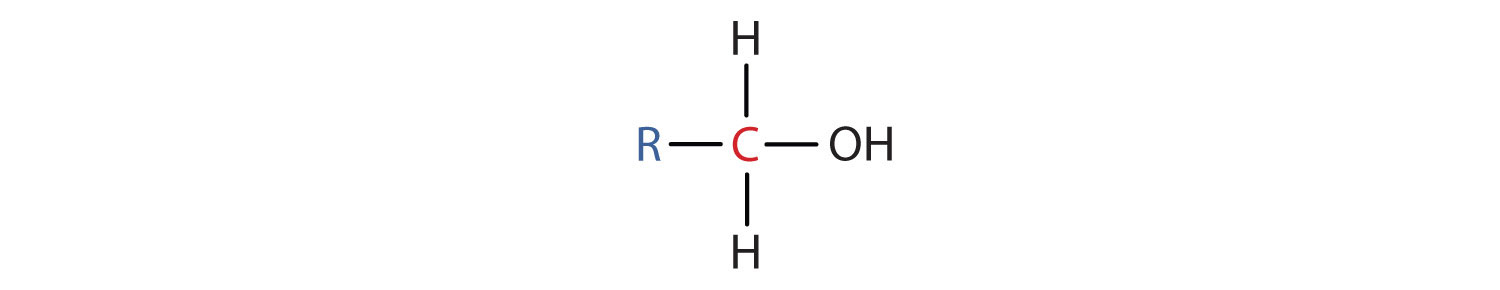
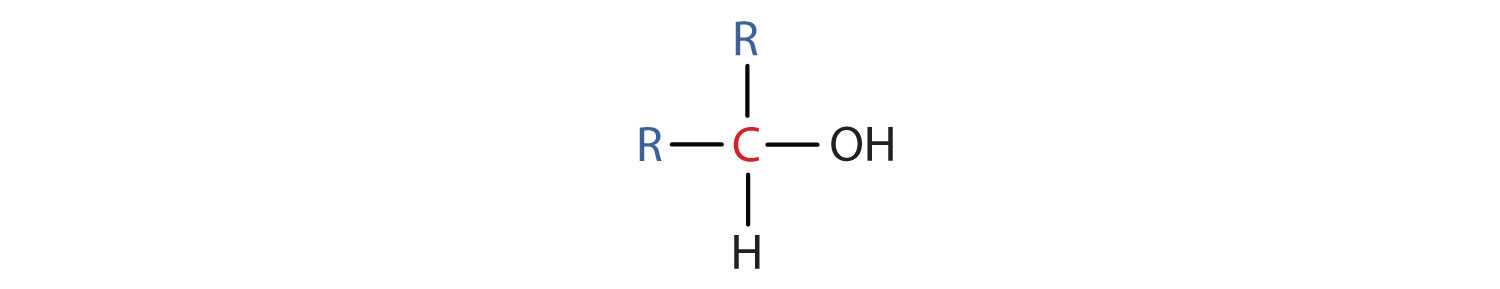
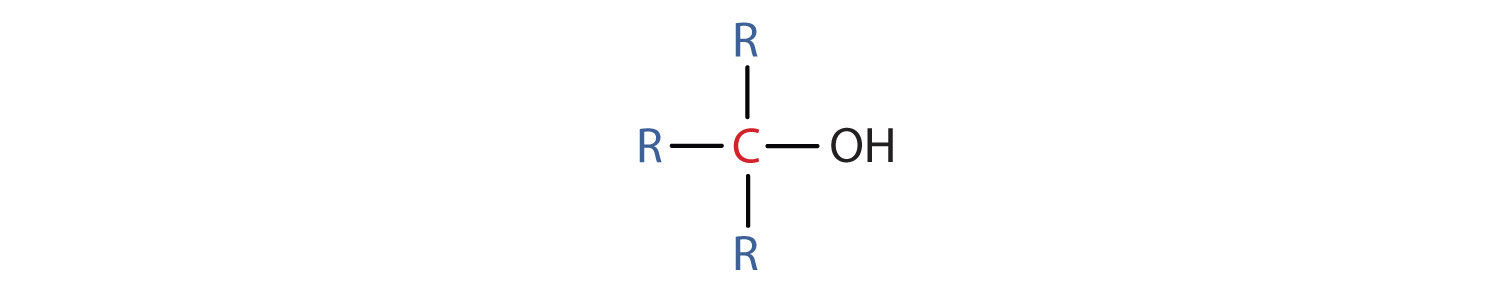
Table 2.2 "Classification and Nomenclature of Some Alcohols" names and classifies some of the simpler alcohols. Some of the common names reflect a compound’s classification as secondary (sec-) or tertiary (tert-). These designations are not used in the IUPAC nomenclature system for alcohols. Note that there are four butyl alcohols in the table, corresponding to the four butyl groups: the butyl group (CH3CH2CH2CH2) introduced in Chapter 1 "Organic Chemistry Review / Hydrocarbons", Section 1.5 "IUPAC Nomenclature", and three others:
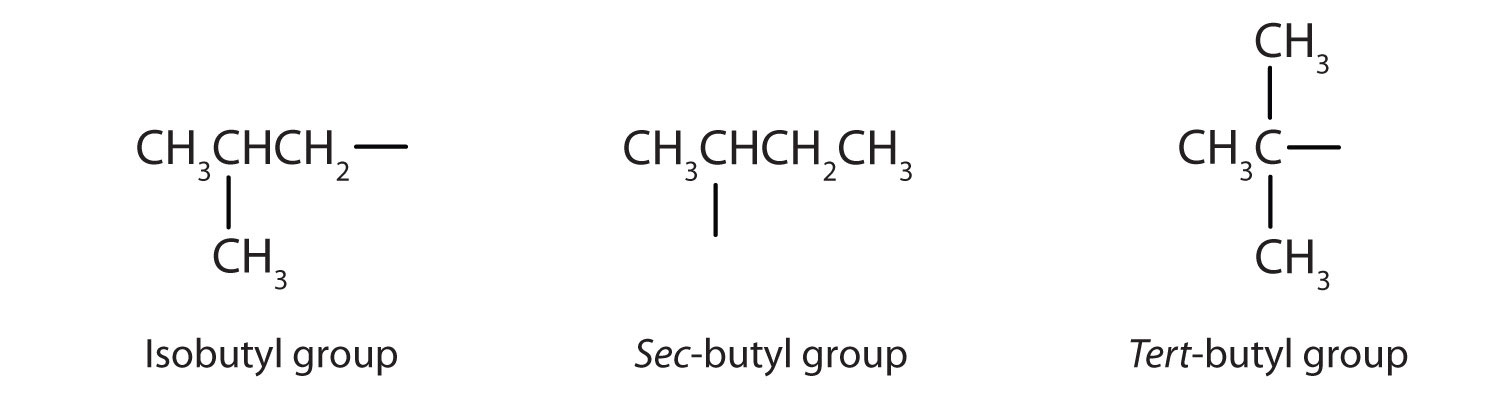
Table 2.2 Classification and Nomenclature of Some Alcohols
| Condensed Structural Formula | Class of Alcohol | Common Name | IUPAC Name |
|---|---|---|---|
| CH3OH | — | methyl alcohol | methanol |
| CH3CH2OH | primary | ethyl alcohol | ethanol |
| CH3CH2CH2OH | primary | propyl alcohol | 1-propanol |
| (CH3)2CHOH | secondary | isopropyl alcohol | 2-propanol |
| CH3CH2CH2CH2OH | primary | butyl alcohol | 1-butanol |
| CH3CH2CHOHCH3 | secondary | sec-butyl alcohol | 2-butanol |
| (CH3)2(CH3)2CHCH2OH | primary | isobutyl alcohol | 2-methyl-1-propanol |
| (CH3)3COH | tertiary | tert-butyl alcohol | 2-methyl-2-propanol |
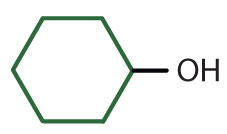 |
secondary | cyclohexyl alcoho | cyclohexanol |
Is isobutyl alcohol primary, secondary, or tertiary? Explain.
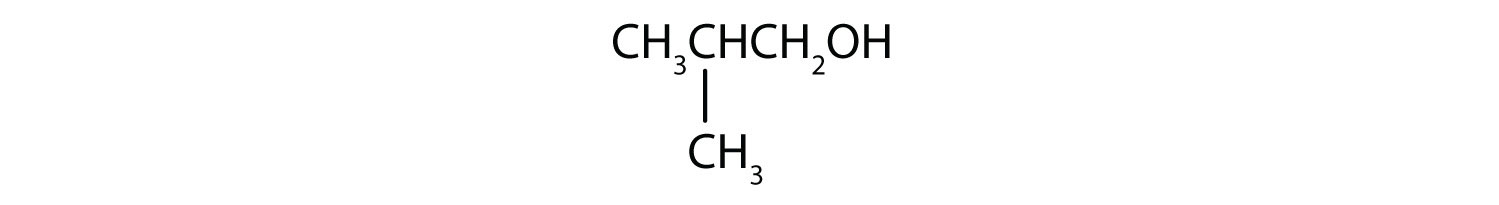
What is the LCC in 2-ethyl-1-hexanol? What is taken as the LCC in naming the compound? Explain.
primary; the carbon atom bearing the OH group is attached to only one other carbon atom
7 carbon atoms; the 6-atom chain includes the carbon atom bearing the OH group
1. Name each alcohol and classify it as primary, secondary, or tertiary.
a. CH3CH2CH2CH2CH2CH2OH
b.
c.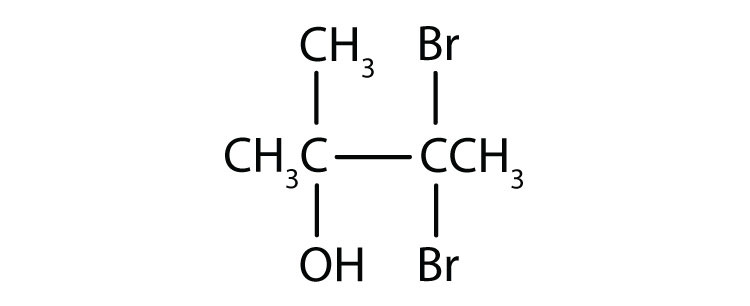
2. Name each alcohol and classify it as primary, secondary, or tertiary.
a.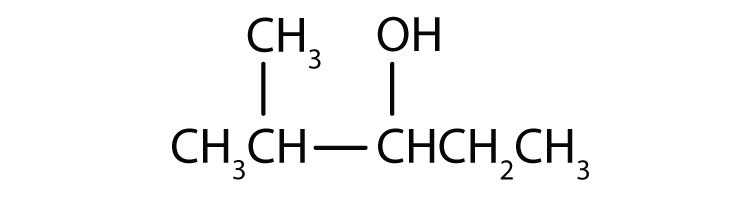
b.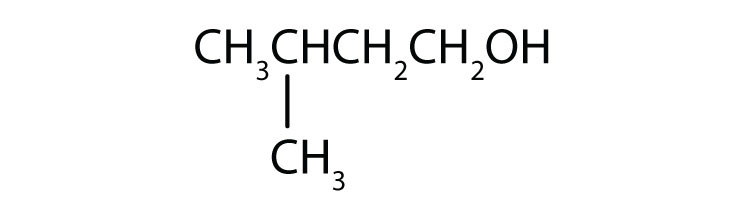
c.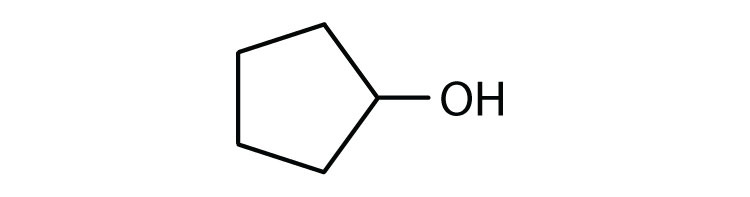
3. Draw the structure for each alcohol.
a. 3-hexanol
b. 3,3-dimethyl-2-butanol
c. cyclobutanol
4. Draw the structure for each alcohol.
a. cyclopentanol
b. 4-methyl-2-hexanol
c. 4,5-dimethyl-3-heptanol
1.
a. 1-hexanol; primary
b. 3-hexanol; secondary
c. 3,3-dibromo-2-methyl-2-butanol; tertiary
3.
a.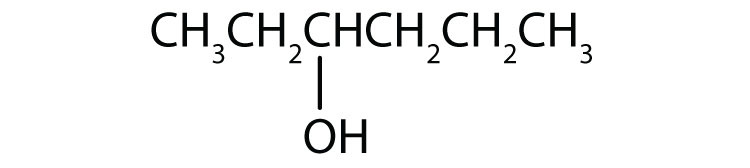
b.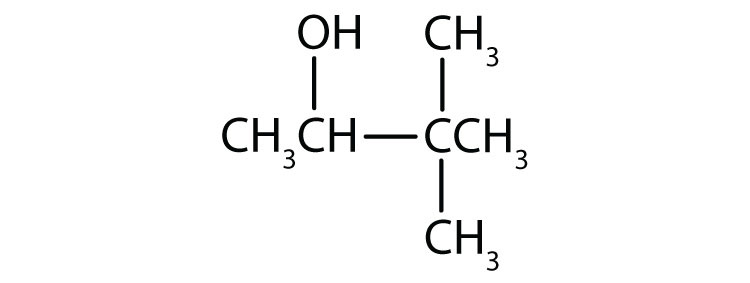
c.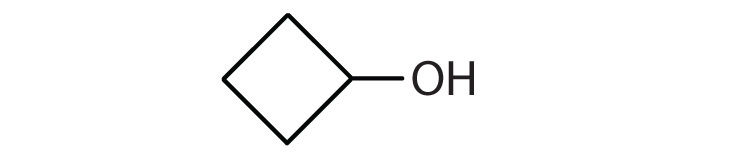
Alcohols can be considered derivatives of water (H2O; also written as HOH).

Like the H–O–H bond in water, the R–O–H bond is bent, and alcohol molecules are polar. This relationship is particularly apparent in small molecules and reflected in the physical and chemical properties of alcohols with low molar mass.
Replacing a hydrogen atom from an alkane with an OH group allows the molecules to associate through hydrogen bonding (Figure 2.2 "Intermolecular Hydrogen Bonding in Methanol"). Remember that physical properties are determined to a large extent by the type of intermolecular forces. Table 2.3 "Comparison of Boiling Points and Molar Masses" lists the molar masses and the boiling points of some common compounds. The table shows that substances with similar molar masses can have quite different boiling points. Alkanes are nonpolar and are thus associated only through relatively weak dispersion forces. Alkanes with one to four carbon atoms are gases at room temperature. In contrast, even methanol (with one carbon atom) is a liquid at room temperature. Hydrogen bonding greatly increases the boiling points of alcohols compared to hydrocarbons of comparable molar mass. The boiling point is a rough measure of the amount of energy necessary to separate a liquid molecule from its nearest neighbors. If the molecules interact through hydrogen bonding, a relatively large quantity of energy must be supplied to break those intermolecular attractions. Only then can the molecule escape from the liquid into the gaseous state.
Figure 2.2 Intermolecular Hydrogen Bonding in Methanol
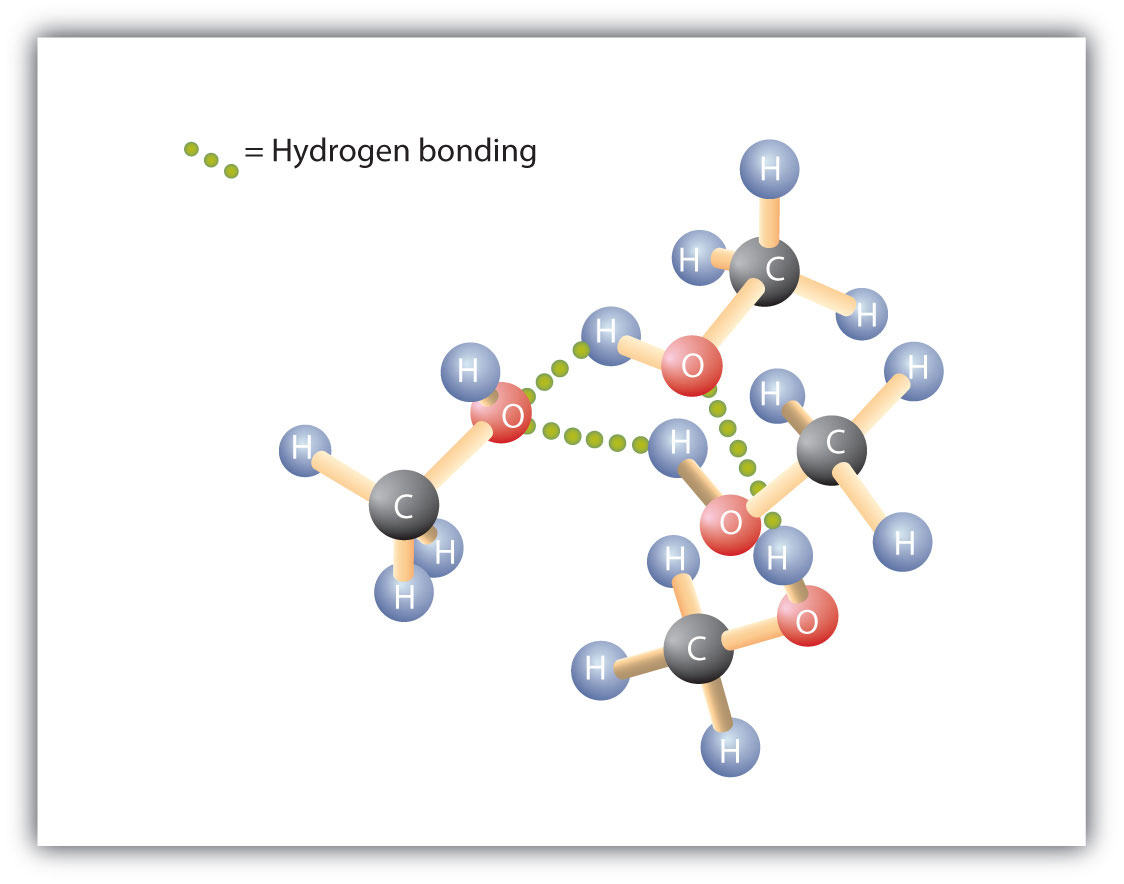
The OH groups of alcohol molecules make hydrogen bonding possible.
Table 2.3 Comparison of Boiling Points and Molar Masses
| Formula | Name | Molar Mass | Boiling Point (°C) |
|---|---|---|---|
| CH4 | methane | 16 | –164 |
| HOH | water | 18 | 100 |
| C2H6 | ethane | 30 | –89 |
| CH3OH | methanol | 32 | 65 |
| C3H8 | propane | 44 | –42 |
| CH3CH2OH | ethanol | 46 | 78 |
| CH3CH2CH2OH | 1-propanol | 60l | 97 |
Alcohols can also engage in hydrogen bonding with water molecules (Figure 2.3 "Hydrogen Bonding between Methanol Molecules and Water Molecules"). Thus, whereas the hydrocarbons are insoluble in water, alcohols with one to three carbon atoms are completely soluble. As the length of the chain increases, however, the solubility of alcohols in water decreases; the molecules become more like hydrocarbons and less like water. The alcohol 1-decanol (CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2OH) is essentially insoluble in water. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon atoms.
Figure 2.3 Hydrogen Bonding between Methanol Molecules and Water Molecules
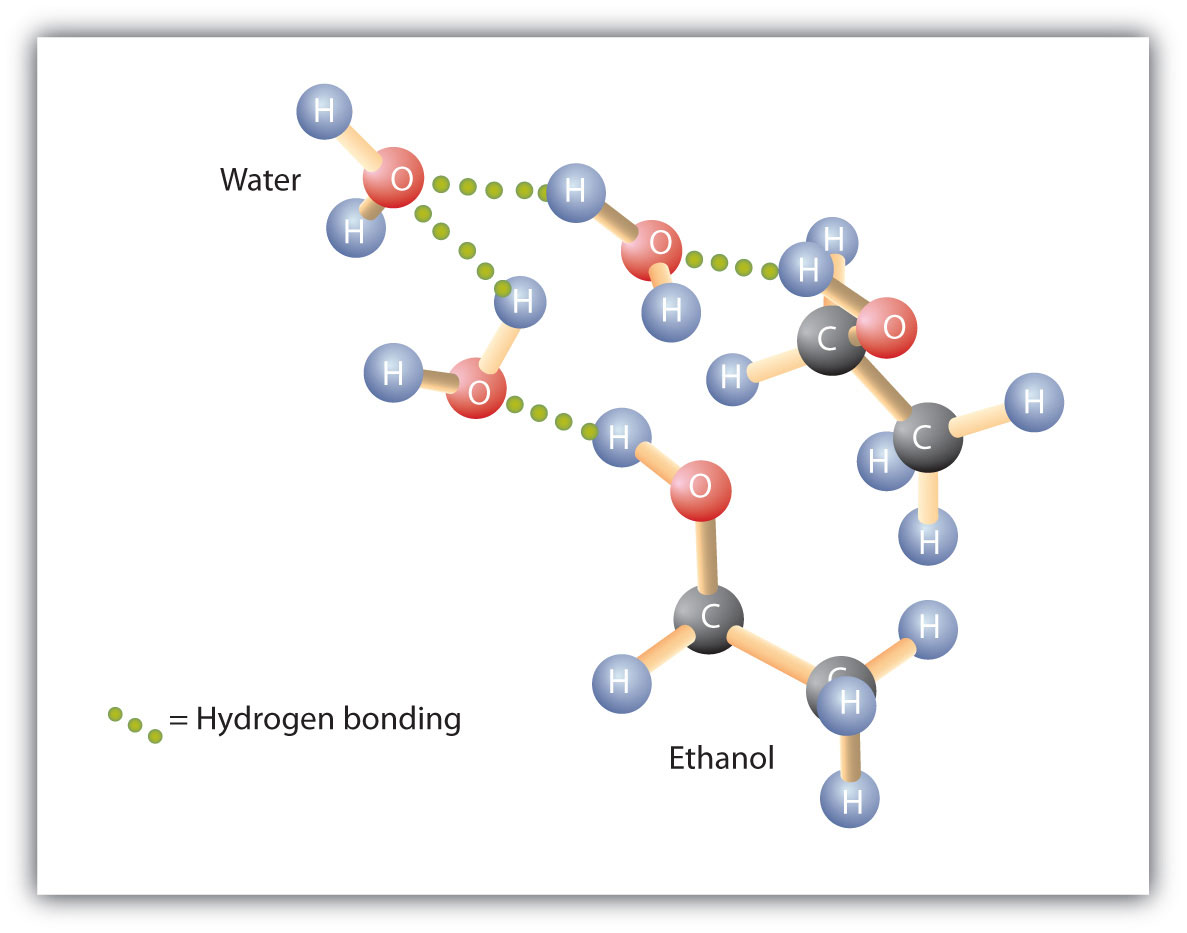
Hydrogen bonding between the OH of methanol and water molecules accounts for the solubility of methanol in water.
Why is ethanol more soluble in water than 1-hexanol?
Why does 1-butanol have a lower boiling point than 1-hexanol?
Ethanol has an OH group and only 2 carbon atoms; 1-hexanol has one OH group for 6 carbon atoms and is thus more like a (nonpolar) hydrocarbon than ethanol is.
The molar mass of 1-hexanol is greater than that of 1-butanol.
Answer the following exercises without consulting tables in the text.
Arrange these alcohols in order of increasing boiling point: ethanol, methanol, and 1-propanol.
Which has the higher boiling point—butane or 1-propanol?
Arrange these alcohols in order of increasing solubility in water: 1-butanol, methanol, and 1-octanol.
Arrange these compounds in order of increasing solubility in water: 1-butanol, ethanol, and pentane.
1. methanol < ethanol < 1-propanol
3. 1-octanol < 1-butanol < methanol
Methanol is prepared by combining hydrogen gas and carbon monoxide at high temperatures and pressures in the presence of a catalyst composed of zinc oxide (ZnO) and chromium oxide (Cr2O3) catalyst:

Methanol is an important solvent and is used as an automotive fuel, either as the pure liquid—as in some racing cars—or as an additive in gasoline.
Nearly 2 billion gallons of methanol are produced each year in the United States by the catalytic reduction of carbon monoxide with hydrogen gas.
Many simple alcohols are made by the hydration of alkenes. (For more information about the hydration of alkenes, see Chapter 1 "Organic Chemistry Review / Hydrocarbons", Section 1.14 "Chemical Properties of Alkenes".) Ethanol is made by the hydration of ethylene in the presence of a catalyst such as sulfuric acid (H2SO4).
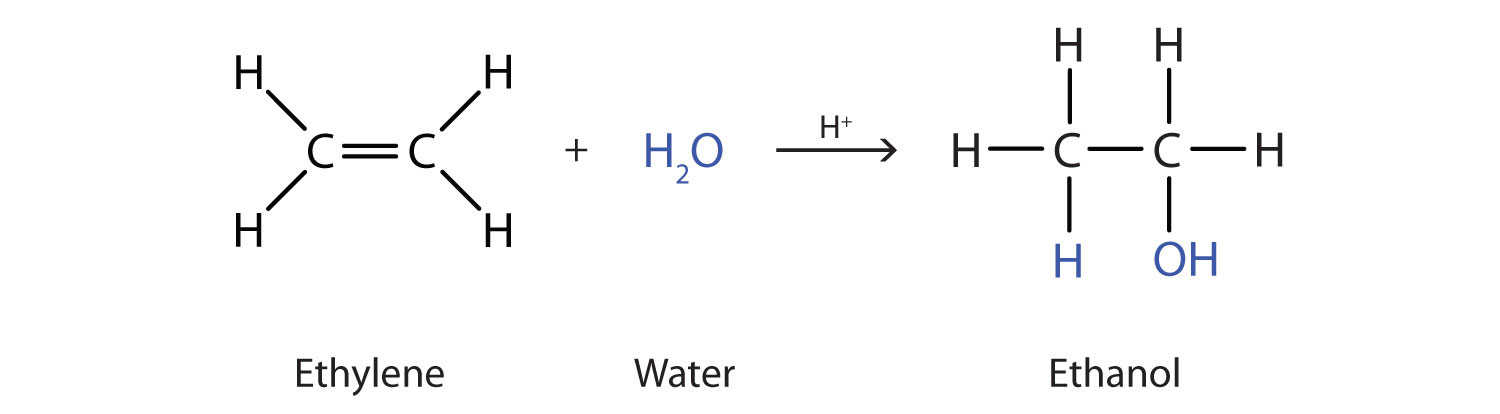
In a similar manner, isopropyl alcohol is produced by the addition of water to propene (propylene).
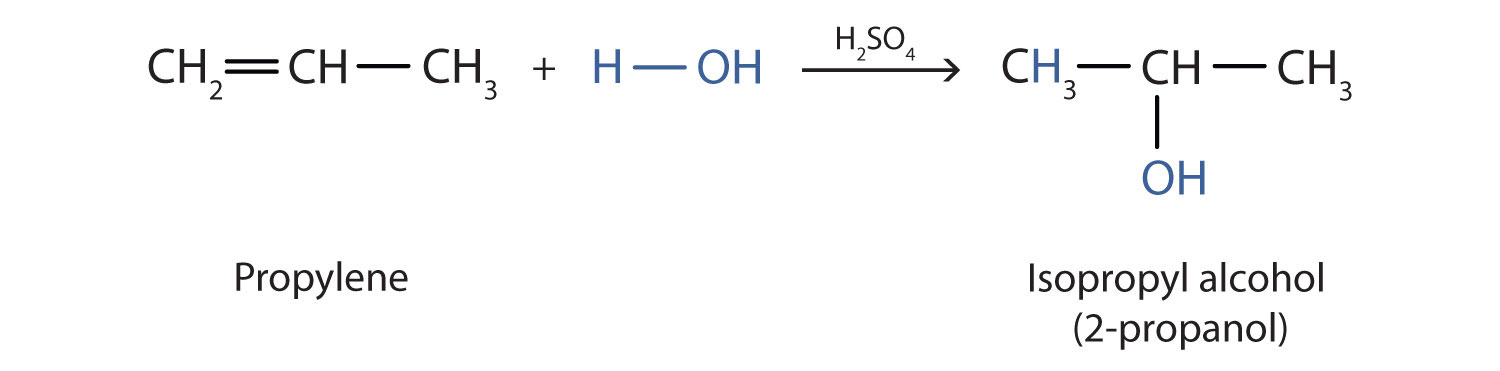
Additional Exercise 19 describes how to use a generalization called Markovnikov’s rule to predict the results when the addition of water to an alcohol has two possible products.
Write the equation for the reaction of 2-butene with water to form 2-butanol. Indicate that sulfuric acid is used as a catalyst.
Solution
First write the condensed structural formula of 2-butene and indicate that it reacts with water. Then write the condensed structural formula of 2-butanol after the reaction arrow to indicate that it is the product. Finally, write the formula for the catalyst above the arrow.
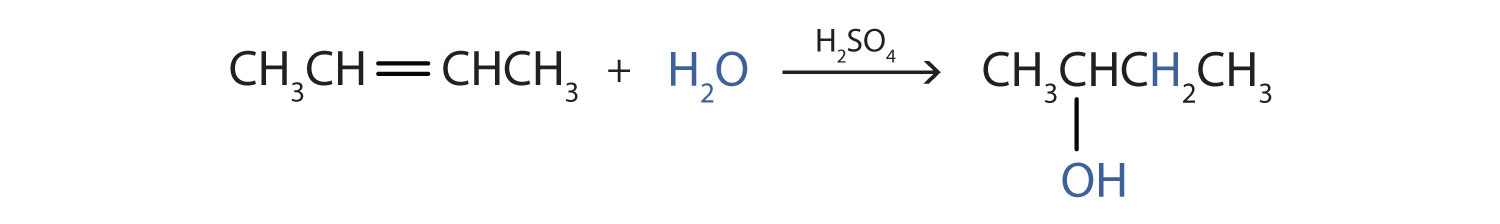
Write the equation for the reaction of cyclopentene with water to form cyclopentanol. Indicate that phosphoric acid (H3PO4) is used as a catalyst.
Many OH compounds in living systems are formed by alkene hydration. Here is an example that occurs in the Krebs cycle: fumarate is hydrated to form malate. (For more information about the Krebs cycle, see Chapter 11 "Metabolic Pathways and Energy Production", Section 11.4 "Stage III of Catabolism".)
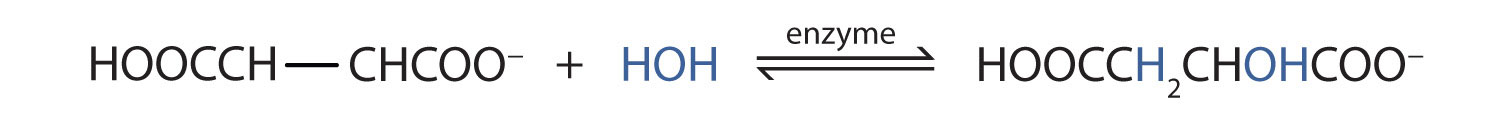
In addition to its preparation from ethylene, ethanol is made by the fermentation of sugars or starch from various sources (potatoes, corn, wheat, rice, etc.). Fermentation is catalyzed by enzymes found in yeast and proceeds by an elaborate multistep mechanism. We can represent the overall process as follows:
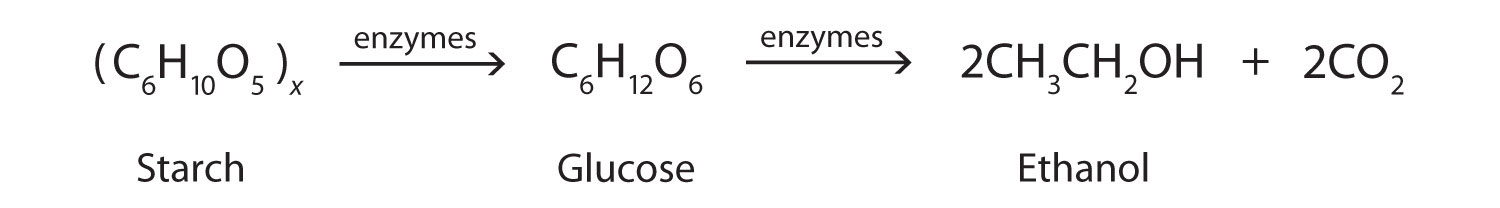
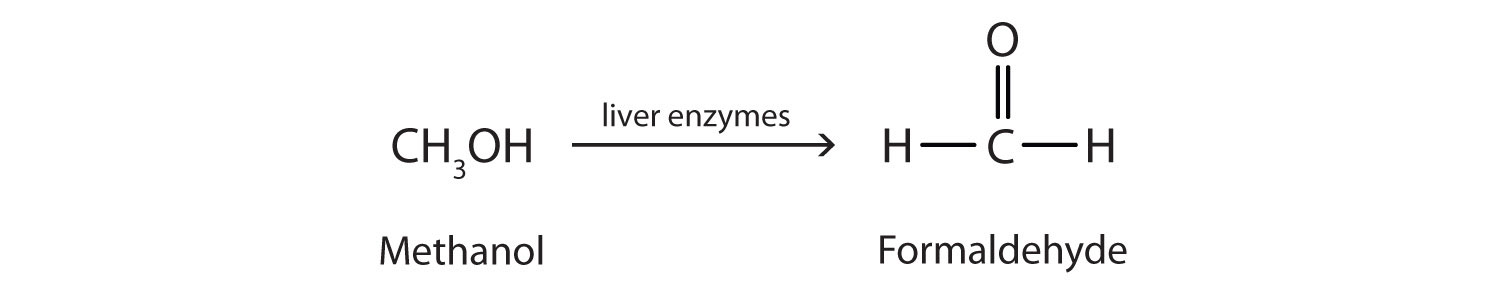
Methanol is quite poisonous to humans. Ingestion of as little as 15 mL of methanol can cause blindness, and 30 mL (1 oz) can cause death. However, the usual fatal dose is 100 to 150 mL. The main reason for methanol’s toxicity is that we have liver enzymes that catalyze its oxidation to formaldehyde, the simplest member of the aldehyde family (See Chapter 3 "Aldehydes, Ketones" Section 3.1 "Aldehydes and Ketones: Structure and Names"):
Formaldehyde reacts rapidly with the components of cells, coagulating proteins in much the same way that cooking coagulates an egg. This property of formaldehyde accounts for much of the toxicity of methanol.
Organic and biochemical equations are frequently written showing only the organic reactants and products. In this way, we focus attention on the organic starting material and product, rather than on balancing complicated equations.
Ethanol is oxidized in the liver to acetaldehyde:
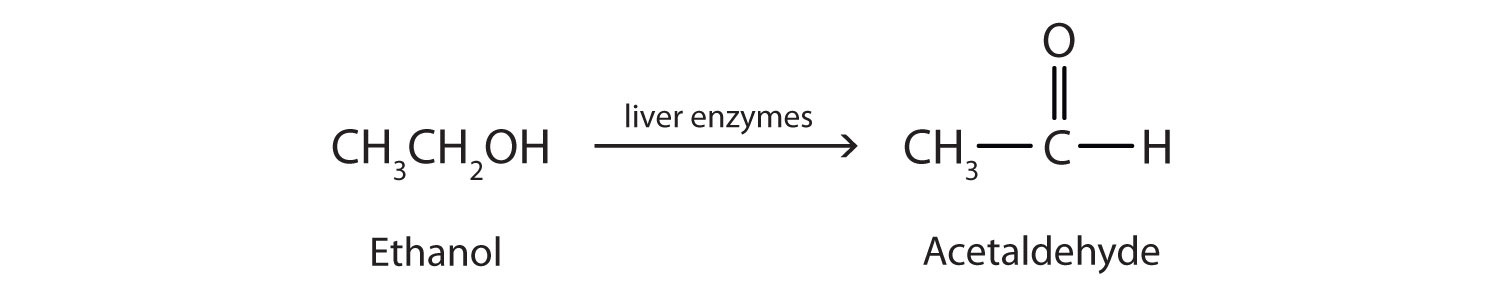
The acetaldehyde is in turn oxidized to acetic acid (HC2H3O2), a normal constituent of cells, which is then oxidized to carbon dioxide and water. Even so, ethanol is potentially toxic to humans. The rapid ingestion of 1 pt (about 500 mL) of pure ethanol would kill most people, and acute ethanol poisoning kills several hundred people each year—often those engaged in some sort of drinking contest. Ethanol freely crosses into the brain, where it depresses the respiratory control center, resulting in failure of the respiratory muscles in the lungs and hence suffocation. Ethanol is believed to act on nerve cell membranes, causing a diminution in speech, thought, cognition, and judgment.
Rubbing alcohol is usually a 70% aqueous solution of isopropyl alcohol. It has a high vapor pressure, and its rapid evaporation from the skin produces a cooling effect. It is toxic when ingested but, compared to methanol, is less readily absorbed through the skin.
Why is methanol more toxic than ethanol?
How does rubbing alcohol cool a feverish patient?
Methanol is oxidized to formaldehyde, which destroys tissue; ethanol is oxidized to acetaldehyde and then acetic acid, a normal metabolite.
Evaporation removes heat.
From what alkene is ethanol made? Draw its condensed structural formula.
Can methanol be made from an alkene? Explain.
ethylene; CH2=CH2
Chemical reactions in alcohols occur mainly at the functional group, but some involve hydrogen atoms attached to the OH-bearing carbon atom or to an adjacent carbon atom. Of the three major kinds of alcohol reactions, which are summarized in Figure 2.4 "Reactions of Alcohols", two—dehydration and oxidation—are considered here. The third reaction type—esterification—is covered in Chapter 4 "Carboxylic Acids, Esters", Section 4.8 "Preparation of Esters".
Figure 2.4 Reactions of Alcohols
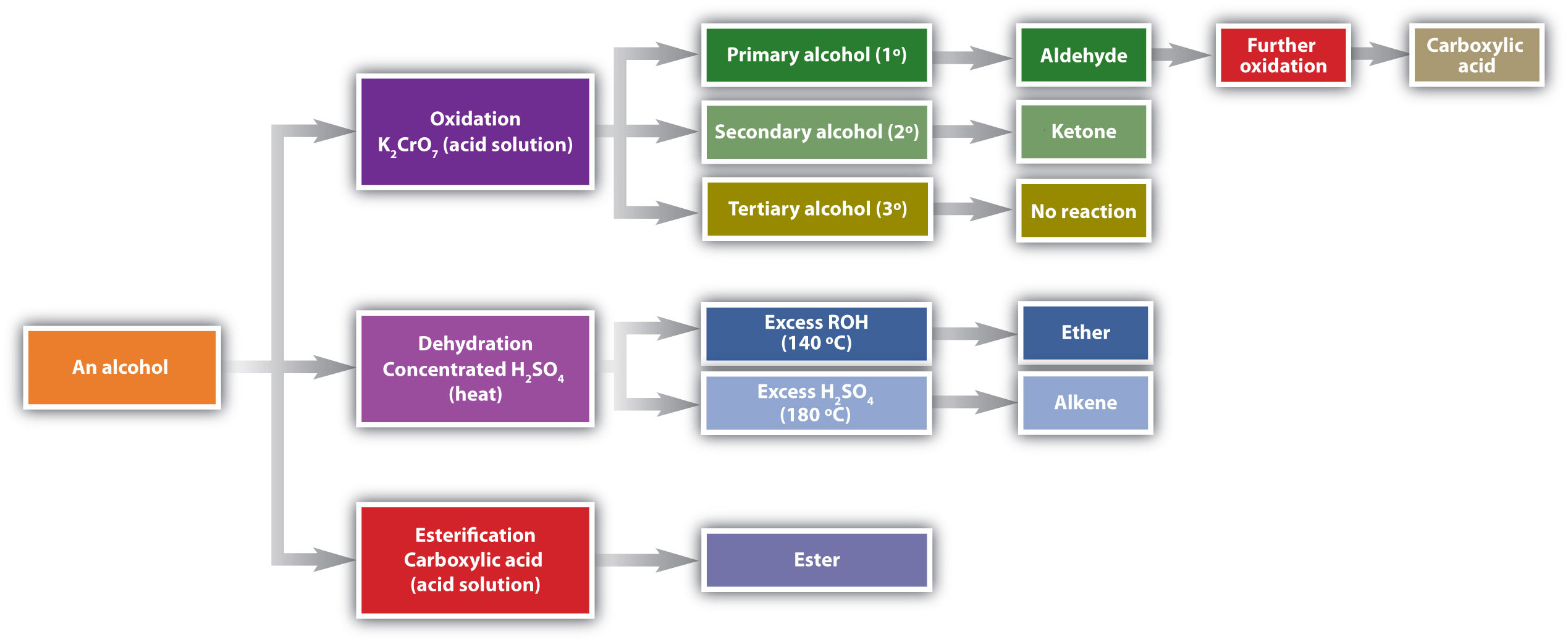
Oxidation and dehydration of alcohols are considered here.
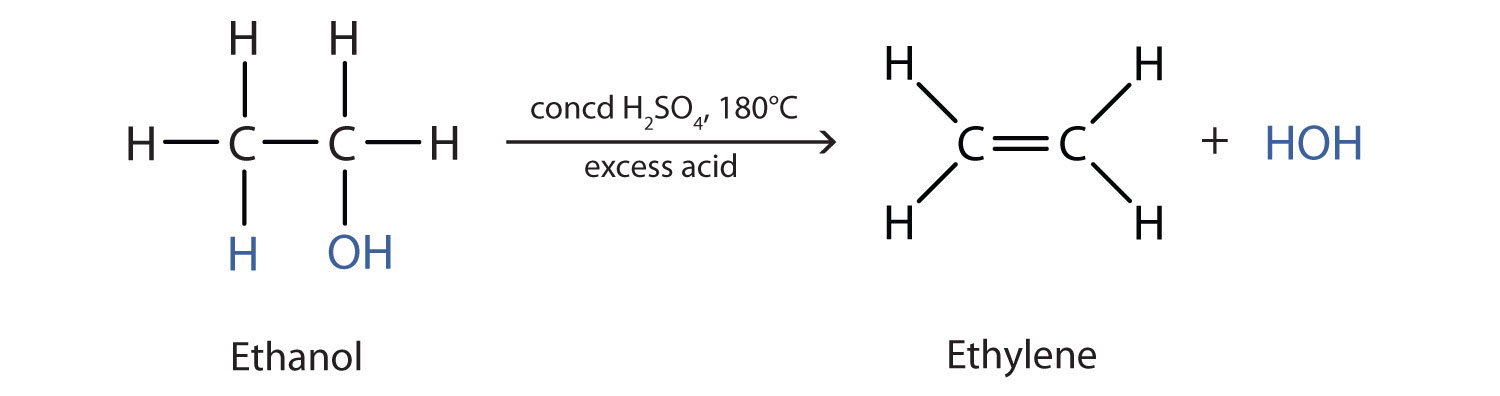
As noted in Figure 2.4 "Reactions of Alcohols", an alcohol undergoes dehydration in the presence of a catalyst to form an alkene and water. The reaction removes the OH group from the alcohol carbon atom and a hydrogen atom from an adjacent carbon atom in the same molecule:
Under the proper conditions, it is possible for the dehydration to occur between two alcohol molecules. The entire OH group of one molecule and only the hydrogen atom of the OH group of the second molecule are removed. The two ethyl groups attached to an oxygen atom form an ether molecule.
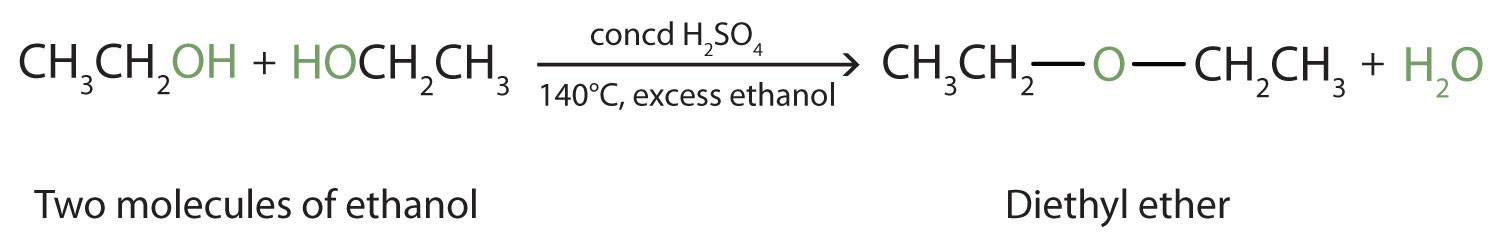
(Ethers are discussed in Section 2.4 "Reactions That Form Alcohols".) Thus, depending on conditions, one can prepare either alkenes or ethers by the dehydration of alcohols.
Both dehydration and hydration reactions occur continuously in cellular metabolism, with enzymes serving as catalysts and at a temperature of about 37°C. (For more information about hydration reactions, see Chapter 1 "Organic Chemistry Review / Hydrocarbons", Section 1.14 "Chemical Properties of Alkenes".) The following reaction occurs in the Embden–Meyerhof pathway. (For more information about metabolic reactions, see Chapter 11 "Metabolic Pathways and Energy Production".)
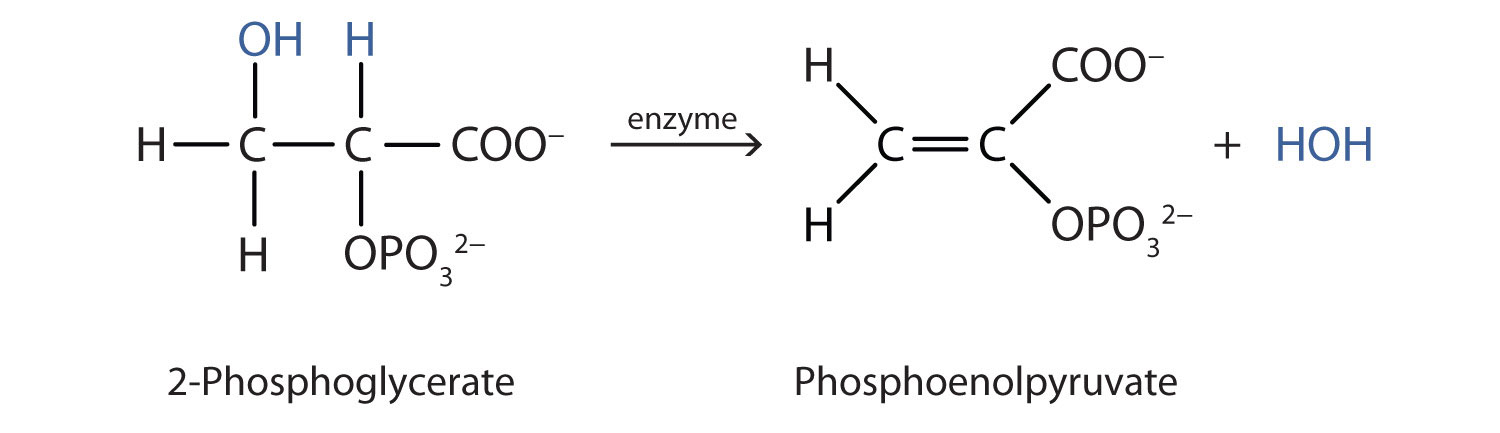
Although the participating compounds are complex, the reaction is the same: elimination of water from the starting material. The idea is that if you know the chemistry of a particular functional group, you know the chemistry of hundreds of different compounds.
Primary and secondary alcohols are readily oxidized. We saw earlier how methanol and ethanol are oxidized by liver enzymes to form aldehydes. Because a variety of oxidizing agents can bring about oxidation, we can indicate an oxidizing agent without specifying a particular one by writing an equation with the symbol [O] above the arrow. For example, we write the oxidation of ethanol—a primary alcohol—to form acetaldehyde—an aldehyde—as follows:
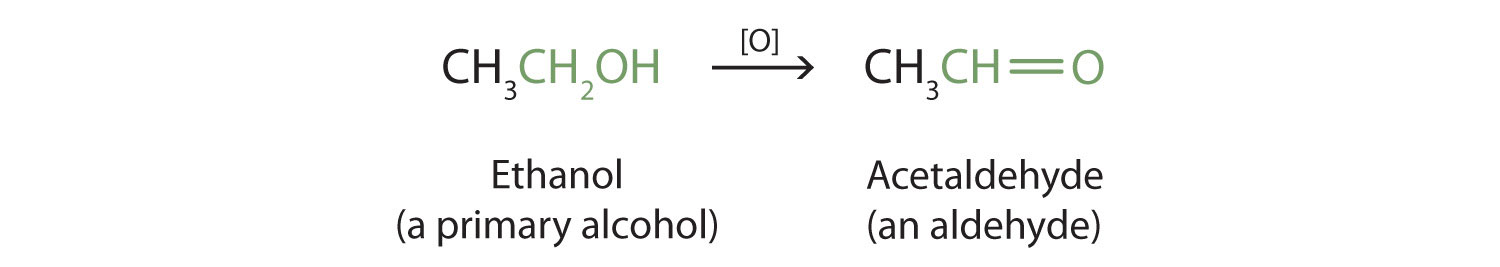
We shall see (in Chapter 3 "Aldehydes, Ketones" Section 3.1 "Aldehydes and Ketones: Structure and Names") that aldehydes are even more easily oxidized than alcohols and yield carboxylic acids.
Secondary alcohols are oxidized to ketones. The oxidation of isopropyl alcohol by potassium dichromate (K2Cr2O7) gives acetone, the simplest ketone:
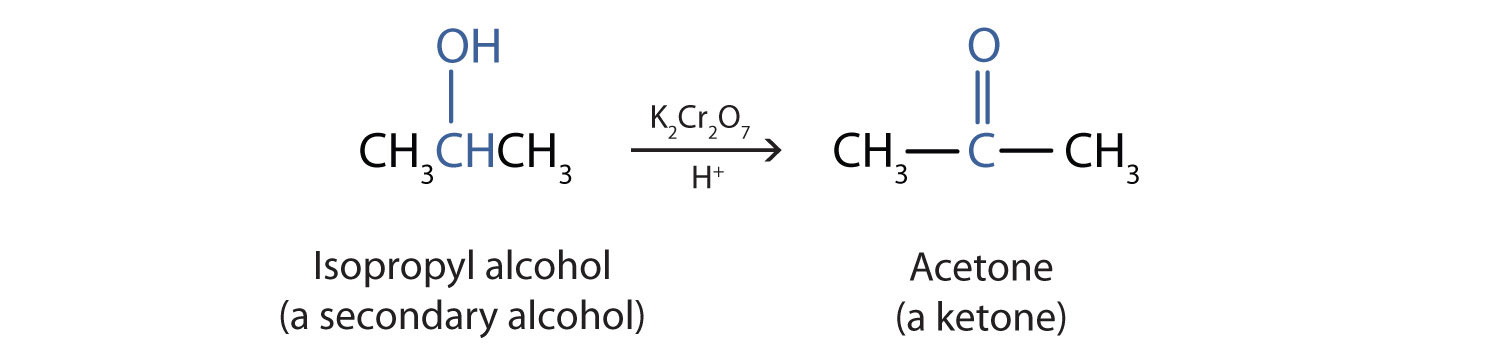
Unlike aldehydes, ketones are relatively resistant to further oxidation (Chapter 3 "Aldehydes, Ketones" Section 3.1 "Aldehydes and Ketones: Structure and Names"), so no special precautions are required to isolate them as they form.
Note that in oxidation of both primary (RCH2OH) and secondary (R2CHOH) alcohols, two hydrogen atoms are removed from the alcohol molecule, one from the OH group and other from the carbon atom that bears the OH group.
These reactions can also be carried out in the laboratory with chemical oxidizing agents. One such oxidizing agent is potassium dichromate. The balanced equation (showing only the species involved in the reaction) in this case is as follows:
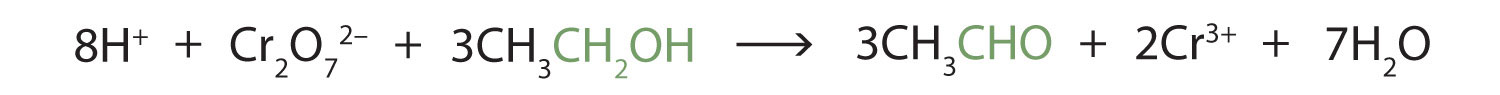
Alcohol oxidation is important in living organisms. Enzyme-controlled oxidation reactions provide the energy cells need to do useful work. One step in the metabolism of carbohydrates involves the oxidation of the secondary alcohol group in isocitric acid to a ketone group:
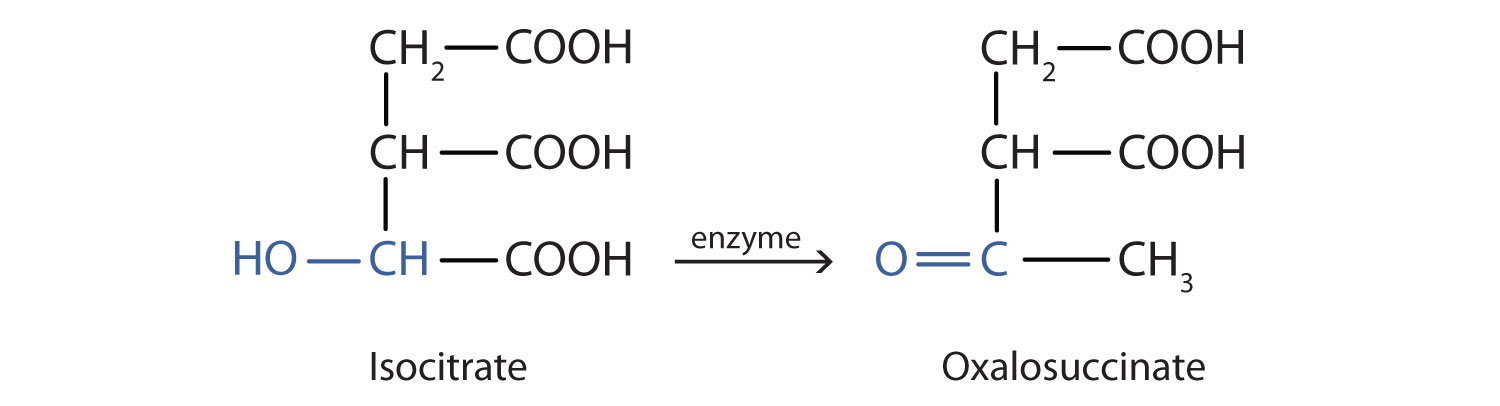
Note that the overall type of reaction is the same as that in the conversion of isopropyl alcohol to acetone. (For more information on metabolic reactions, see Chapter 11 "Metabolic Pathways and Energy Production".)
Tertiary alcohols (R3COH) are resistant to oxidation because the carbon atom that carries the OH group does not have a hydrogen atom attached but is instead bonded to other carbon atoms. The oxidation reactions we have described involve the formation of a carbon-to-oxygen double bond. Thus, the carbon atom bearing the OH group must be able to release one of its attached atoms to form the double bond. The carbon-to-hydrogen bonding is easily broken under oxidative conditions, but carbon-to-carbon bonds are not. Therefore tertiary alcohols are not easily oxidized.
Write an equation for the oxidation of each alcohol. Use [O] above the arrow to indicate an oxidizing agent. If no reaction occurs, write “no reaction” after the arrow.
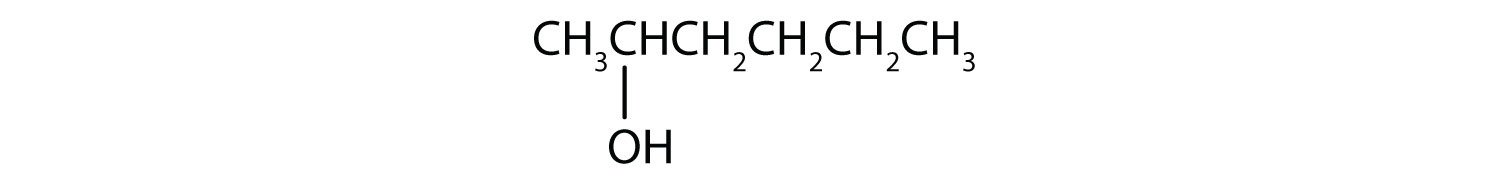
Solution
The first step is to recognize the class of each alcohol as primary, secondary, or tertiary.
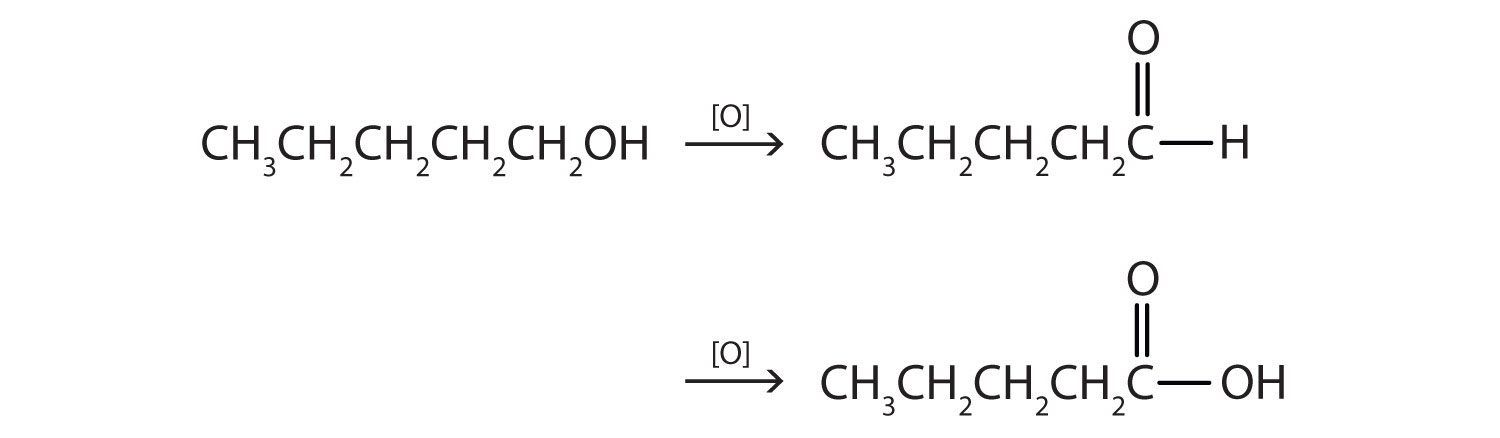
This alcohol has the OH group on a carbon atom that is attached to only one other carbon atom, so it is a primary alcohol. Oxidation forms first an aldehyde and further oxidation forms a carboxylic acid.
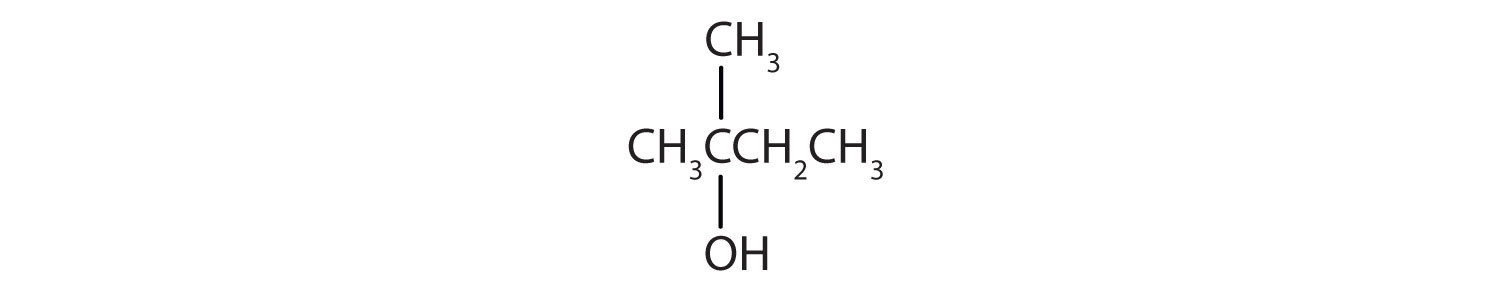
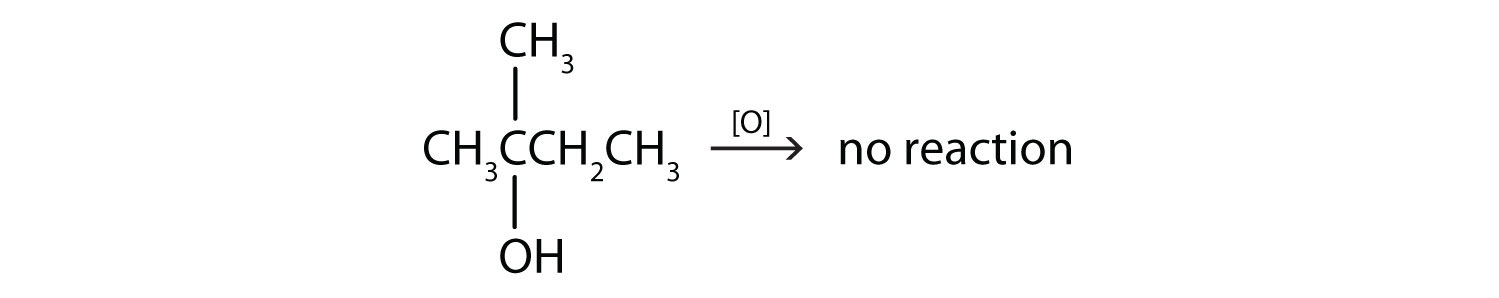
This alcohol has the OH group on a carbon atom that is attached to three other carbon atoms, so it is a tertiary alcohol. No reaction occurs.
This alcohol has the OH group on a carbon atom that is attached to two other carbon atoms, so it is a secondary alcohol; oxidation gives a ketone.
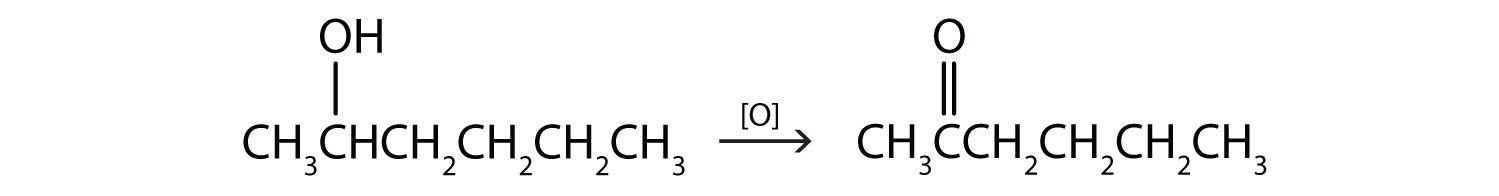
Write an equation for the oxidation of each alcohol. Use [O] above the arrow to indicate an oxidizing agent. If no reaction occurs, write “no reaction” after the arrow.
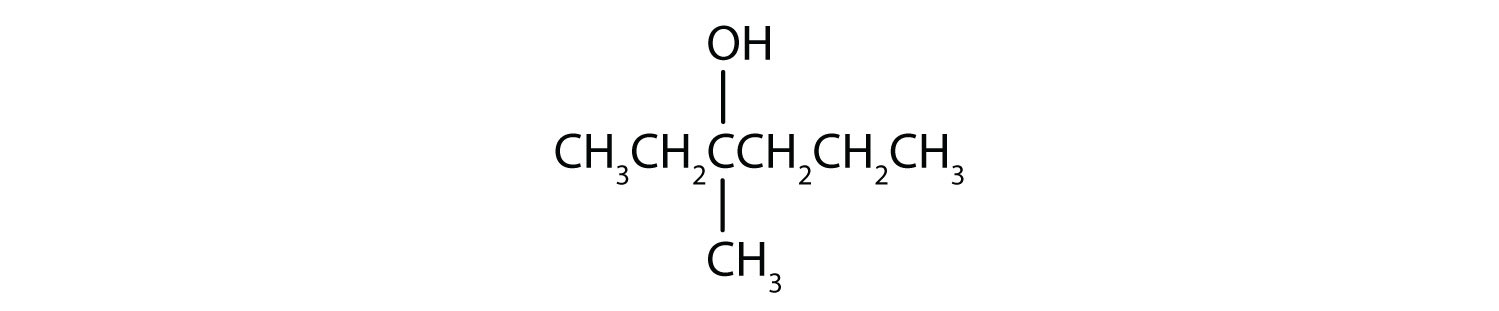
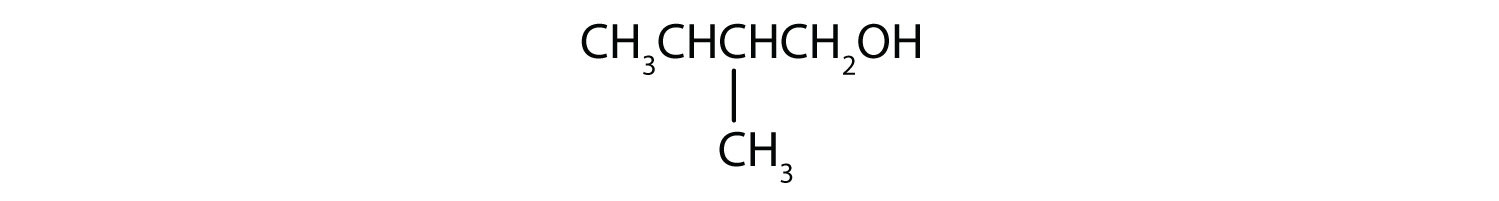
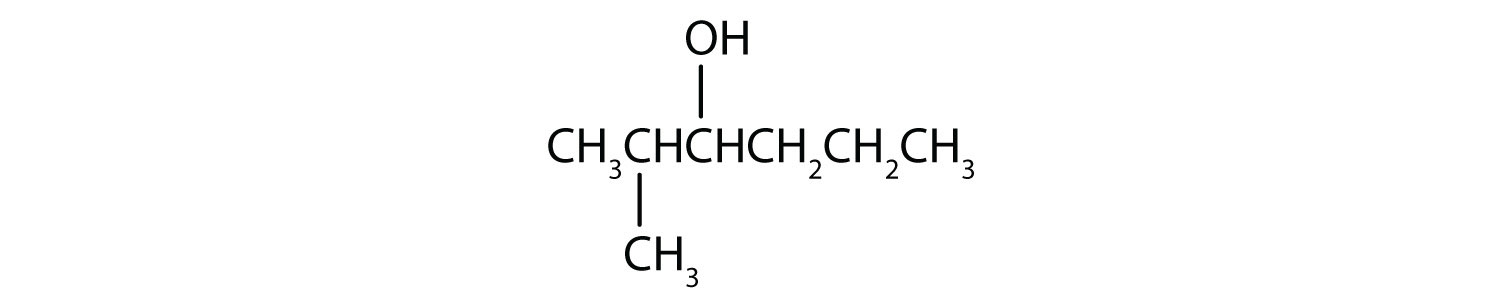
In a reaction, compound W with the molecular formula C4H10O is converted to compound X with the formula C4H8O. Is W oxidized, reduced, dehydrated, or none of these? Explain.
In a reaction, 2 mol of compound Y with the molecular formula C4H10O is converted to 1 mol of compound Z with the formula C8H18O. Is Y oxidized, reduced, or neither? Explain.
oxidized; H is removed
neither; water is removed
1. Name the three major types of chemical reactions of alcohols.
2. Why do tertiary alcohols not undergo oxidation? Can a tertiary alcohol undergo dehydration?
3. Draw the structure of the product for each reaction.
a.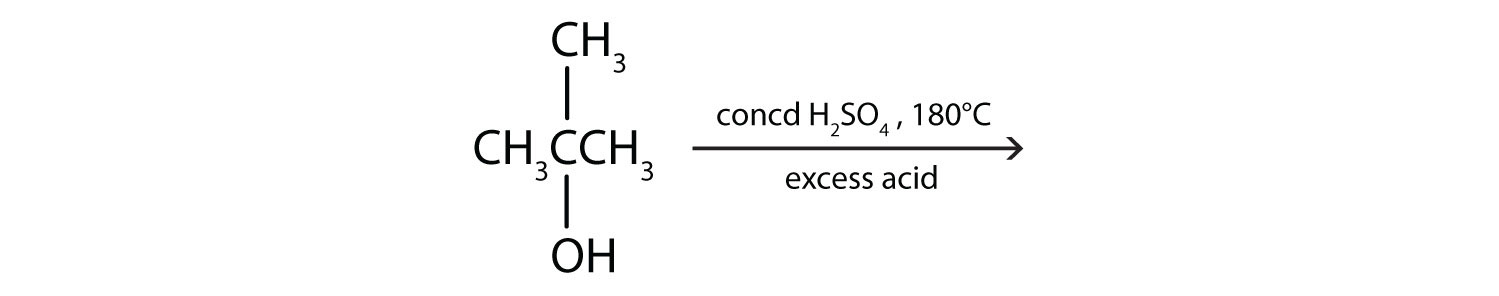
b.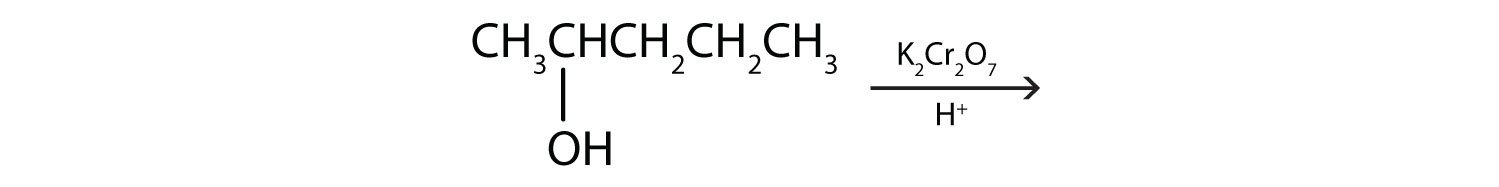
4. Draw the structure of the product for each reaction.
a.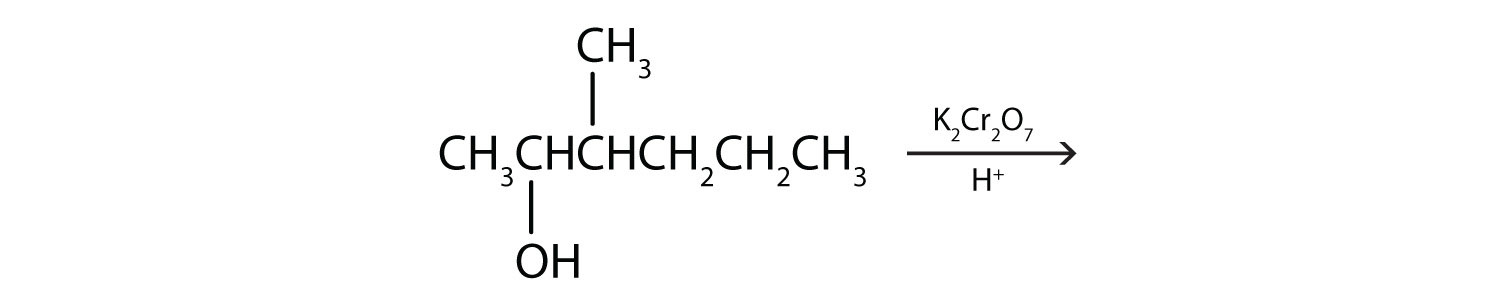
b.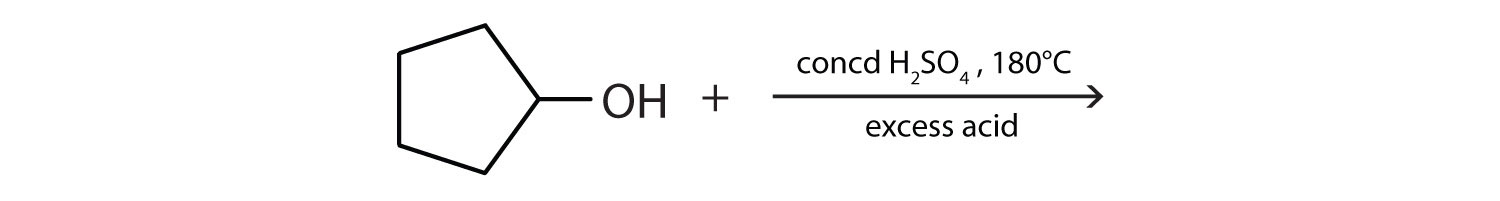 \
\
5. Write an equation for the dehydration of 2-propanol to yield each compound type.
a. an alkene
b. an ether
6. Draw the structure of the alkene formed by the dehydration of cyclohexanol.
1. dehydration, oxidation, and esterification
3.
a.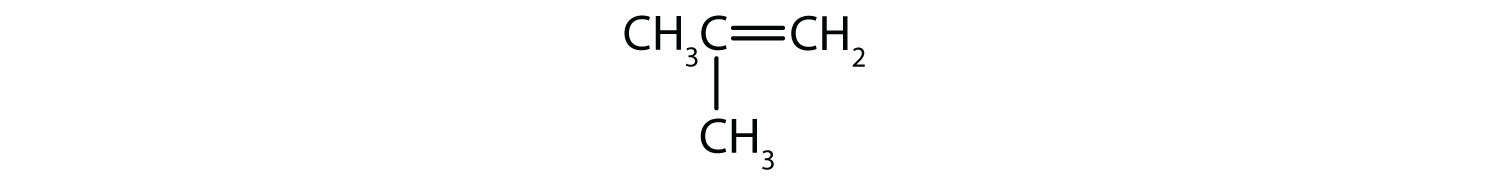
b.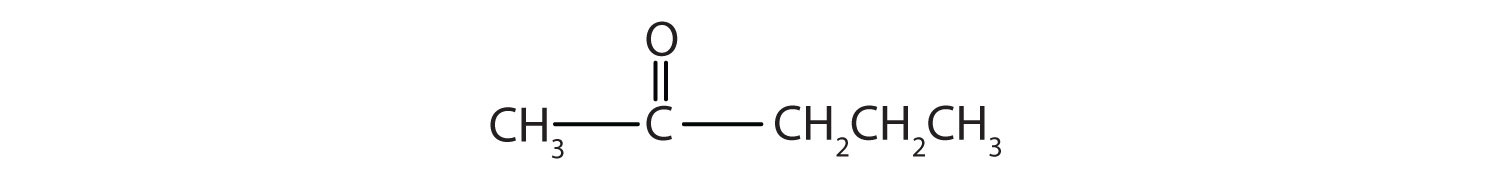
5.
a.
b.
Alcohols with two OH groups on adjacent carbon atoms are commonly known as glycols. The most important of these is 1,2-ethanediol (the common name is ethylene glycol), a sweet, colorless, somewhat viscous liquid.
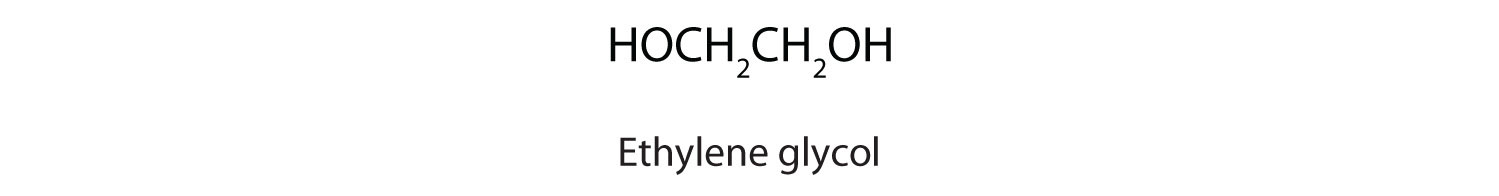
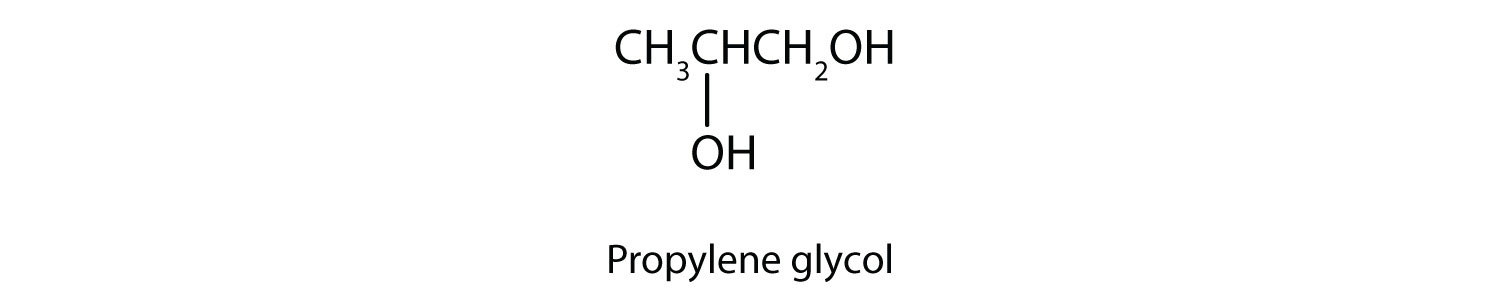
Another common glycol, 1,2-propanediol, is commonly called propylene glycol. Its physical properties are quite similar to those of ethylene glycol.
Commonly called glycerol or glycerin, 1,2,3-propanetriol is the most important trihydroxy alcohol. Like the two glycols, it is a sweet, syrupy liquid. Glycerol is a product of the hydrolysis of fats and oils. (For more information about fats and oils, see Chapter 7 "Lipids", Section 7.2 "Fats and Oils".)
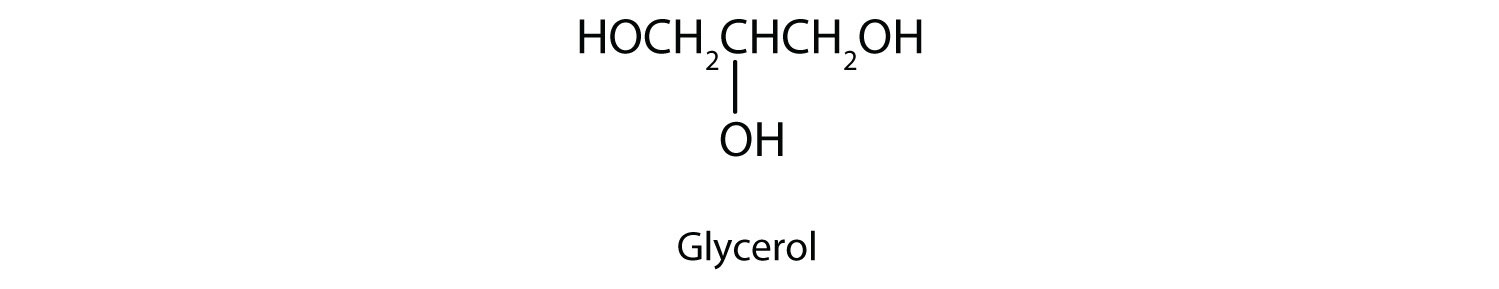
Ethylene glycol is the main ingredient in many antifreeze mixtures for automobile radiators. The two OH groups lead to extensive intermolecular hydrogen bonding. This results in a high boiling point—198°C; thus ethylene glycol does not boil away when it is used as an antifreeze. It is also completely miscible with water. A solution of 60% ethylene glycol in water freezes at −49°C (−56°F) and thus protects an automobile radiator down to that temperature. Ethylene glycol is also used in the manufacture of polyester fiber and magnetic film used in tapes for recorders and computers.
Ethylene glycol is quite toxic. Because it is sweet, pets often lap up spills of leaked antifreeze from a garage floor or driveway. Sometimes people, especially children, drink it. As with methanol, its toxicity is due to a metabolite. Liver enzymes oxidize ethylene glycol to oxalate ion.
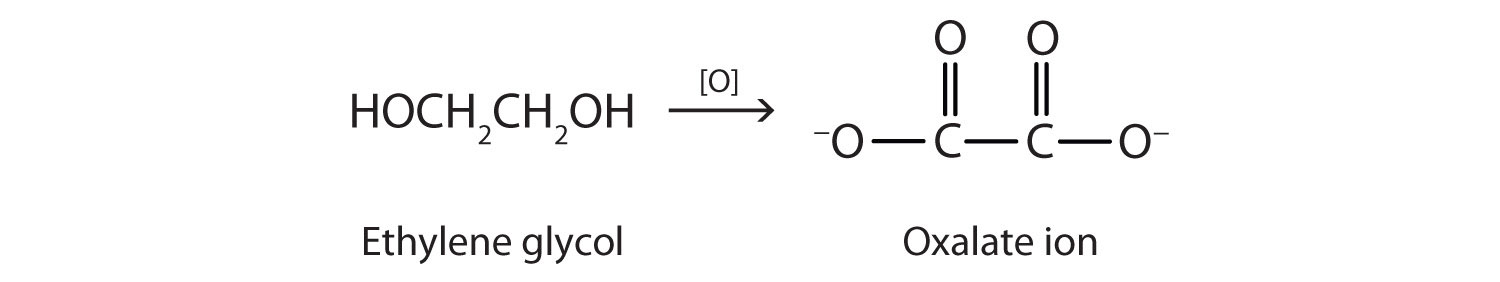
In the kidneys, the oxalate ion combines with the calcium (Ca2+) ion, precipitating as calcium oxalate (CaC2O4).
Ca2+(aq) + C2O42−(aq) → CaC2O4(s)These crystals cause renal damage and can lead to kidney failure and death.
Although propylene glycol has physical properties much like those of ethylene glycol, its physiological properties are quite different. Propylene glycol is essentially nontoxic, and it can be used as a solvent for drugs and as a moisturizing agent for foods. Like other alcohols, propylene glycol is oxidized by liver enzymes.
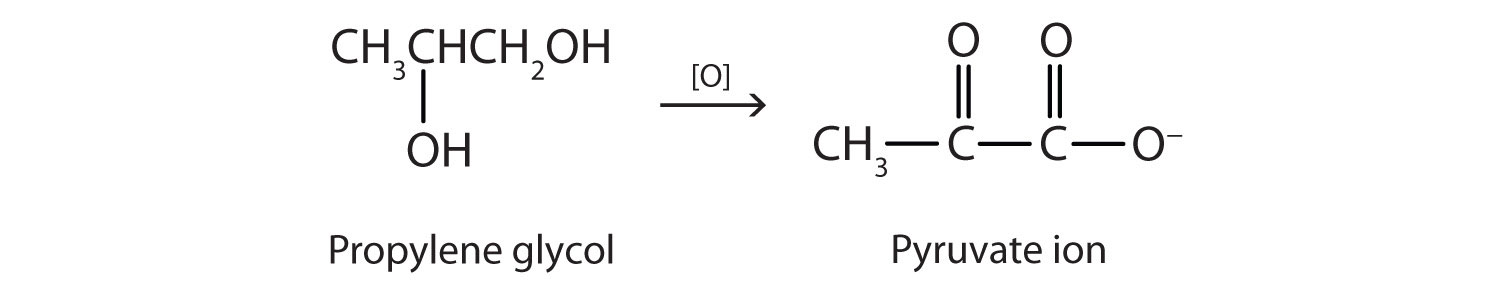
In this case, however, the product is pyruvate ion, a normal intermediate in carbohydrate metabolism. (For more information about metabolic reactions, see Chapter 11 "Metabolic Pathways and Energy Production".)
Glycerol, a product of fat metabolism, is essentially nontoxic.
In the oxidation of propylene glycol to pyruvic acid, what functional groups in the reactant are involved? What new functional groups appear in the product?
Oxalate ion is formed by the oxidation of ethylene glycol. In what kind of reaction is the oxalate ion involved?
two OH groups; a ketone group and a carboxylic acid group
precipitation
1. What is a glycol?
2. Why is ethylene glycol so much more toxic to humans than propylene glycol?
3. Draw the structure for each compound.
a. 1,5-pentanediol
b. propylene glycol
4. Draw the structure for each compound.
a. 1,3-hexandiol
b. glycerol
1. an alcohol with two OH groups on adjacent carbon atoms
3.
a. HOCH2CH2CH2CH2CH2OH
b.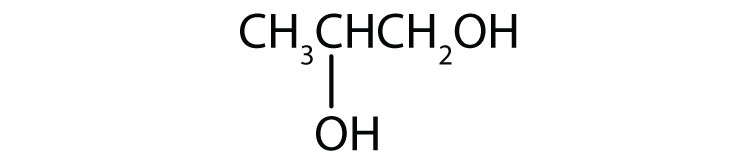
Compounds in which an OH group is attached directly to an aromatic ring are designated ArOH and called phenols. Phenols differ from alcohols in that they are slightly acidic in water. They react with aqueous sodium hydroxide (NaOH) to form salts.
ArOH(aq) + NaOH(aq) → ArONa(aq) + H2O
The parent compound, C6H5OH, is itself called phenol. (An old name, emphasizing its slight acidity, was carbolic acid.) Phenol is a white crystalline compound that has a distinctive (“hospital smell”) odor.
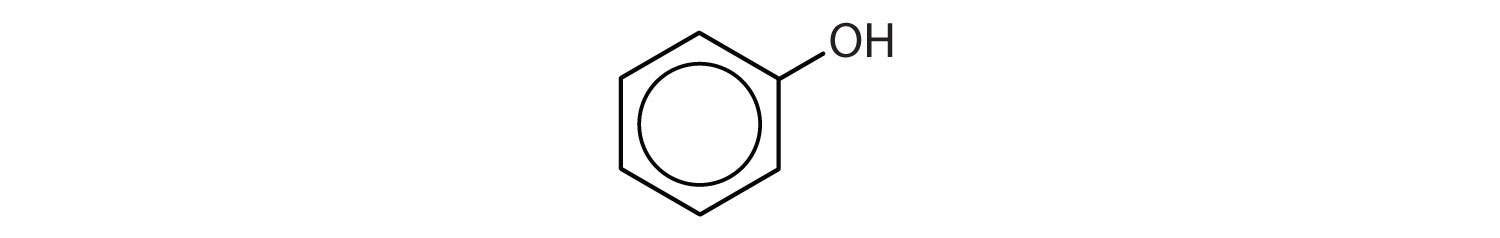
Phenols are widely used as antiseptics (substances that kill microorganisms on living tissue) and as disinfectants (substances intended to kill microorganisms on inanimate objects such as furniture or floors). The first widely used antiseptic was phenol. Joseph Lister used it for antiseptic surgery in 1867. Phenol is toxic to humans, however, and can cause severe burns when applied to the skin. In the bloodstream, it is a systemic poison—that is, one that is carried to and affects all parts of the body. Its severe side effects led to searches for safer antiseptics, a number of which have been found.
One safer phenolic antiseptic is 4-hexylresorcinol (4-hexyl-1,3-dihydroxybenzene; resorcinol is the common name for 1,3-dihydroxybenzene, and 4-hexylresorcinol has a hexyl group on the fourth carbon atom of the resorcinol ring). It is much more powerful than phenol as a germicide and has fewer undesirable side effects. Indeed, it is safe enough to be used as the active ingredient in some mouthwashes and throat lozenges.
How do phenols differ from alcohols in terms of structure and properties?
How do phenols differ in properties from aromatic hydrocarbons?
Phenols have an OH group attached directly to an aromatic ring. Phenols are weakly acidic.
Phenols have an OH group and are somewhat soluble in water.
1. Name each compound.
a.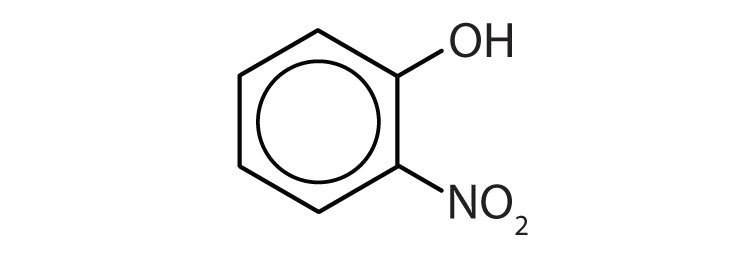
b.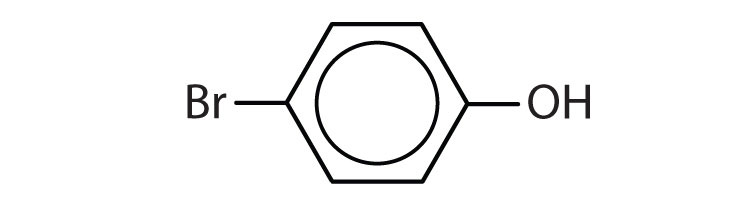
2. Name each compound.
a.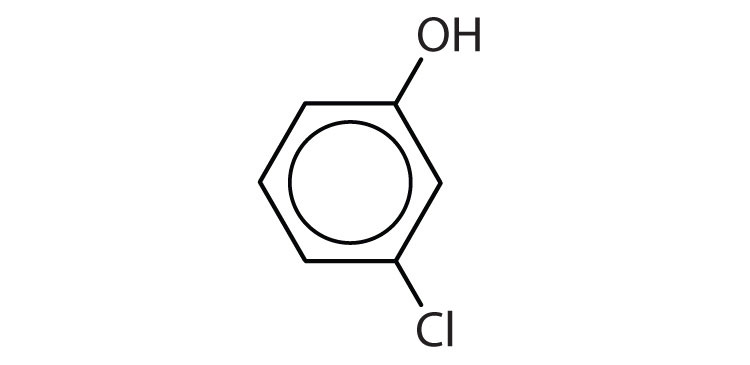
b.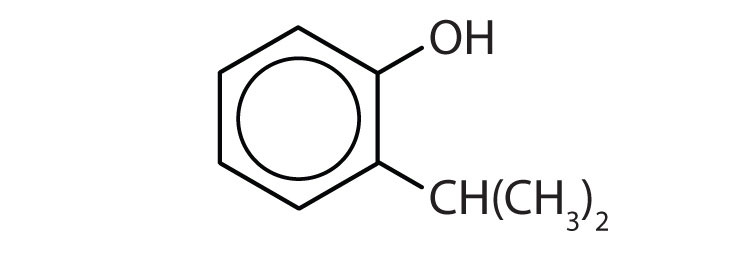
3. Draw the structure for each compound.
a. m-iodophenol
b. p-methylphenol (p-cresol)
4. Draw the structure for each compound.
a. 2,4,6-trinitrophenol (picric acid)
b. 3,5-diethylphenol
1.
a. o-nitrophenol
b. p-bromophenol
3.
a.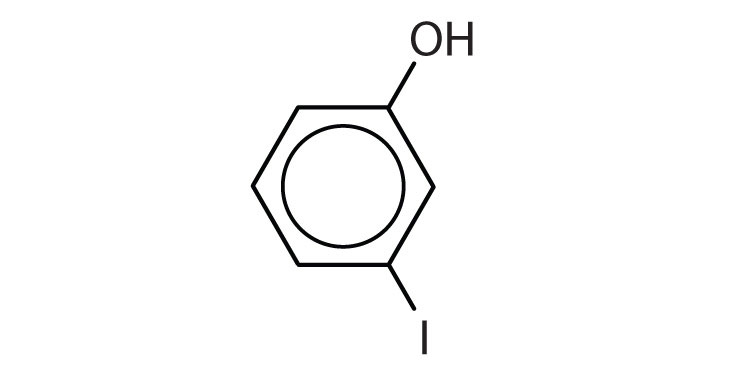
b.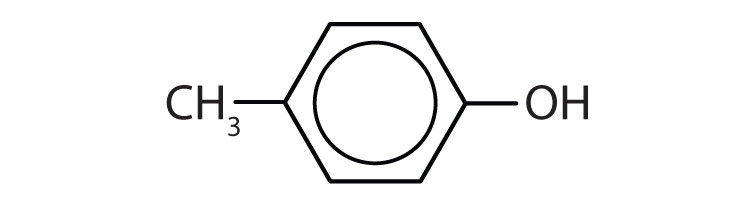
With the general formula ROR′, an ether may be considered a derivative of water in which both hydrogen atoms are replaced by alkyl or aryl groups. It may also be considered a derivative of an alcohol (ROH) in which the hydrogen atom of the OH group is been replaced by a second alkyl or aryl group:

Simple ethers have simple common names, formed from the names of the groups attached to oxygen atom, followed by the generic name ether. For example, CH3–O–CH2CH2CH3 is methyl propyl ether. If both groups are the same, the group name should be preceded by the prefix di-, as in dimethyl ether (CH3–O–CH3) and diethyl ether CH3CH2–O–CH2CH3.
Ether molecules have no hydrogen atom on the oxygen atom (that is, no OH group). Therefore there is no intermolecular hydrogen bonding between ether molecules, and ethers therefore have quite low boiling points for a given molar mass. Indeed, ethers have boiling points about the same as those of alkanes of comparable molar mass and much lower than those of the corresponding alcohols (Table 2.4 "Comparison of Boiling Points of Alkanes, Alcohols, and Ethers").
Table 2.4 Comparison of Boiling Points of Alkanes, Alcohols, and Ethers
| Condensed Structural Formula | Name | Molar Mass | Boiling Point (°C) | Intermolecular Hydrogen Bonding in Pure Liquid? |
|---|---|---|---|---|
| CH3CH2CH3 | propane | 44 | –42 | no |
| CH3OCH3 | dimethyl ether | 46 | –25 | no |
| CH3CH2OH | ethyl alcohol | 46 | 78 | yes |
| CH3CH2CH2CH2CH3 | pentane | 72 | 36 | no |
| CH3CH2OCH2CH3 | diethyl ether | 74 | 35 | no |
| CH3CH2CH2CH2OH | butyl alcohol | 74 | 117 | yes |
Ether molecules do have an oxygen atom, however, and engage in hydrogen bonding with water molecules. Consequently, an ether has about the same solubility in water as the alcohol that is isomeric with it. For example, dimethyl ether and ethanol (both having the molecular formula C2H6O) are completely soluble in water, whereas diethyl ether and 1-butanol (both C4H10O) are barely soluble in water (8 g/100 mL of water).
What is the common name for each ether?
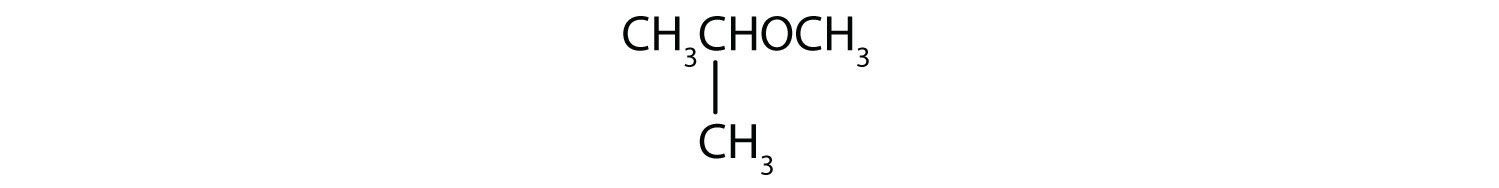
Solution
What is the common name for each ether?
CH3CH2CH2CH2OCH2CH2CH2CH3
A general anesthetic acts on the brain to produce unconsciousness and a general insensitivity to feeling or pain. Diethyl ether (CH3CH2OCH2CH3) was the first general anesthetic to be used.
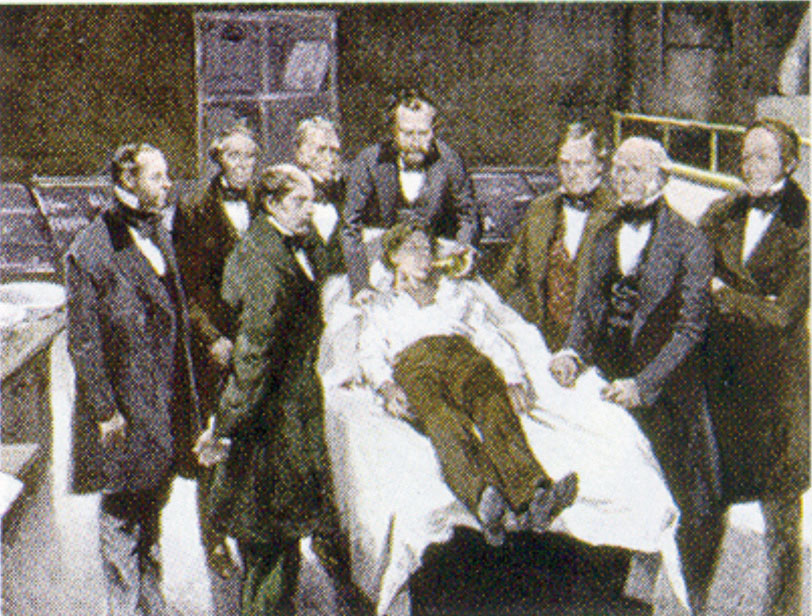
William Morton, a Boston dentist, introduced diethyl ether into surgical practice in 1846. This painting shows an operation in Boston in 1846 in which diethyl ether was used as an anesthetic. Inhalation of ether vapor produces unconsciousness by depressing the activity of the central nervous system.
Source: Painting of William Morton by Ernest Board, from http://commons.wikimedia.org/wiki/File:Morton_Ether_1846.jpg.
Diethyl ether is relatively safe because there is a fairly wide gap between the dose that produces an effective level of anesthesia and the lethal dose. However, because it is highly flammable and has the added disadvantage of causing nausea, it has been replaced by newer inhalant anesthetics, including the fluorine-containing compounds halothane, enflurane, and isoflurane. Unfortunately, the safety of these compounds for operating room personnel has been questioned. For example, female operating room workers exposed to halothane suffer a higher rate of miscarriages than women in the general population.
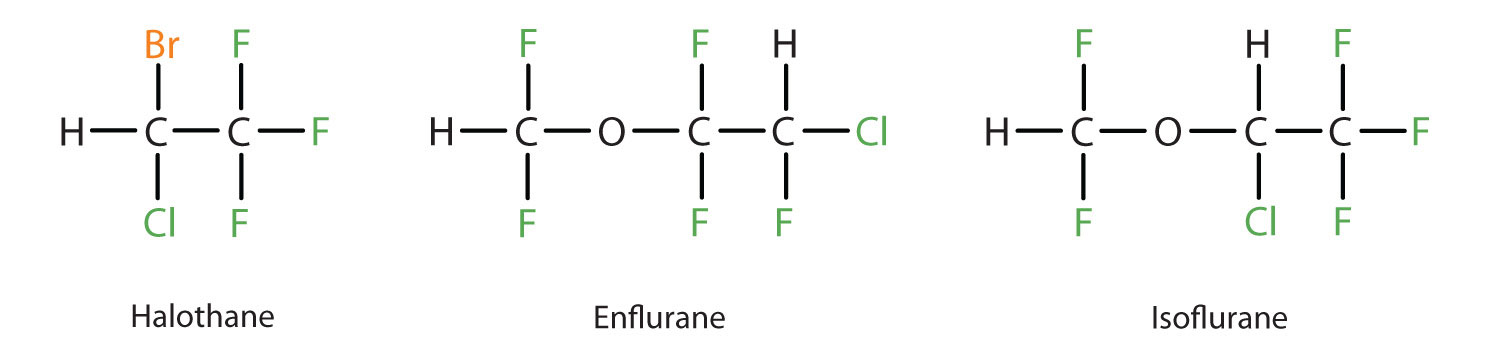
These three modern, inhalant, halogen-containing, anesthetic compounds are less flammable than diethyl ether.
Why does diethyl ether (CH3CH2OCH2CH3) have a much lower boiling point than 1-butanol (CH3CH2CH2CH2OH)?
Which is more soluble in water—ethyl methyl ether (CH3CH2OCH3) or 1-butanol (CH3CH2CH2CH2OH)? Explain.
Diethyl ether has no intermolecular hydrogen bonding because there is no OH group; 1-butanol has an OH and engages in intermolecular hydrogen bonding.
Ethyl methyl ether (three carbon atoms, one oxygen atom) is more soluble in water than 1-butanol (four carbon atoms, one oxygen atom), even though both can engage in hydrogen bonding with water.
1. How can ethanol give two different products when heated with sulfuric acid? Name these products.
2. Which of these ethers is isomeric with ethanol—CH3CH2OCH2CH3, CH3OCH2CH3, or CH3OCH3?
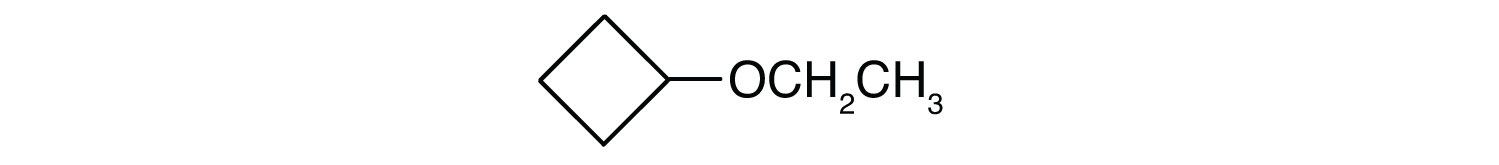
3. Name each compound.
a. CH3OCH2CH2CH3
b.
4. Name each compound.
a. CH3CH2CH2CH2OCH3
b. CH3CH2OCH2CH2CH3
5. Draw the structure for each compound.
a. methyl ethyl ether
b. tert-butyl ethyl ether
6. Draw the structure for each compound.
a. diisopropyl ether
b. cyclopropyl propyl ether
1. Intramolecular (both the H and the OH come from the same molecule) dehydration gives ethylene; intermolecular (the H comes from one molecule and the OH comes from another molecule) dehydration gives diethyl ether.
3.
a. methyl propyl ether
b. ethyl isopropyl ether
5.
a. CH3OCH2CH3
b.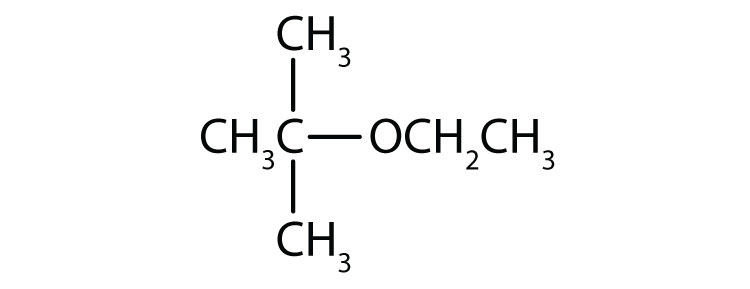
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
