Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball, John W. Hill, and Rhonda J. Scott
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
Organic acids have been known for ages. Prehistoric people likely made acetic acid when their fermentation reactions went awry and produced vinegar instead of wine. The Sumerians (2900–1800 BCE) used vinegar as a condiment, a preservative, an antibiotic, and a detergent. Citric acid was discovered by an Islamic alchemist, Jabir Ibn Hayyan (also known as Geber), in the 8th century, and crystalline citric acid was first isolated from lemon juice in 1784 by the Swedish chemist Carl Wilhelm Scheele. Medieval scholars in Europe were aware that the crisp, tart flavor of citrus fruits is caused by citric acid. Naturalists of the 17th century knew that the sting of a red ant’s bite was due to an organic acid that the ant injected into the wound. The acetic acid of vinegar, the formic acid of red ants, and the citric acid of fruits all belong to the same family of compounds—carboxylic acids. Soaps are salts of long-chain carboxylic acids. (For more information about soaps, see Chapter 7 "Lipids", Section 7.2 "Fats and Oils".)
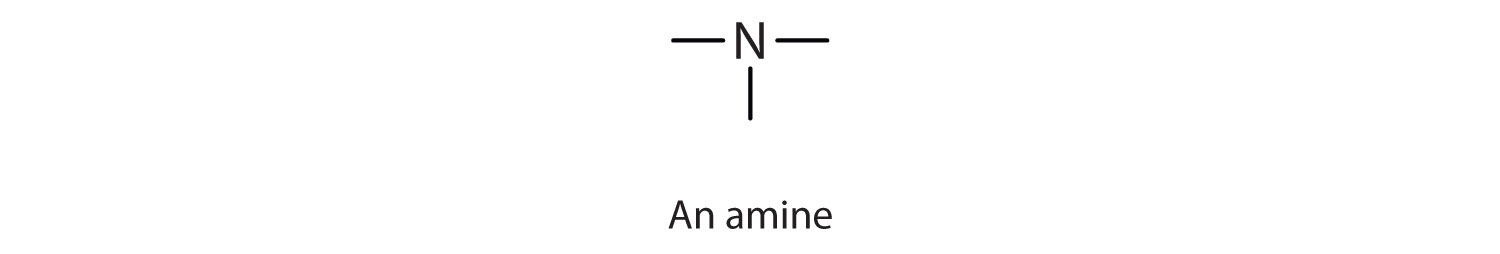
Prehistoric people also knew about organic bases—by smell if not by name; amines are the organic bases produced when animal tissue decays.
The organic compounds that we consider in this chapter are organic acids and bases. We will also consider two derivatives of carboxylic acids: esters and amides. An ester is derived from a carboxylic acid and an alcohol. Fats and oils are esters, as are many important fragrances and flavors. (For more information about fats and oils, see Chapter 7 "Lipids", Section 7.2 "Fats and Oils".) An amide is derived from a carboxylic acid and either ammonia or an amine. Proteins, often called “the stuff of life,” are polyamides. (For more information about proteins, see Chapter 9 "Proteins, and Enzymes", Section 9.1 "Proteins".)
We introduced the carbonyl group (C=O)—the functional group of aldehydes and ketones—in Chapter 3 "Aldehydes, Ketones". The carbonyl group is also found in carboxylic acids, esters, and amides. However, in these compounds, the carbonyl group is only part of the functional group.
A carboxylic acid is an organic compound that has a carboxyl group. The carboxyl group is a functional group that contains a carbon–oxygen double bond and an OH group also attached to the same carbon atom, but it has characteristic properties of its own. As with aldehydes and ketones, carboxylic acid formulas can be written to show the carbon-to-oxygen double bond explicitly, or the carboxyl group can be written in condensed form on one line. In general, carboxylic acids are represented by the formula RCOOH, where R is a hydrocarbon group.
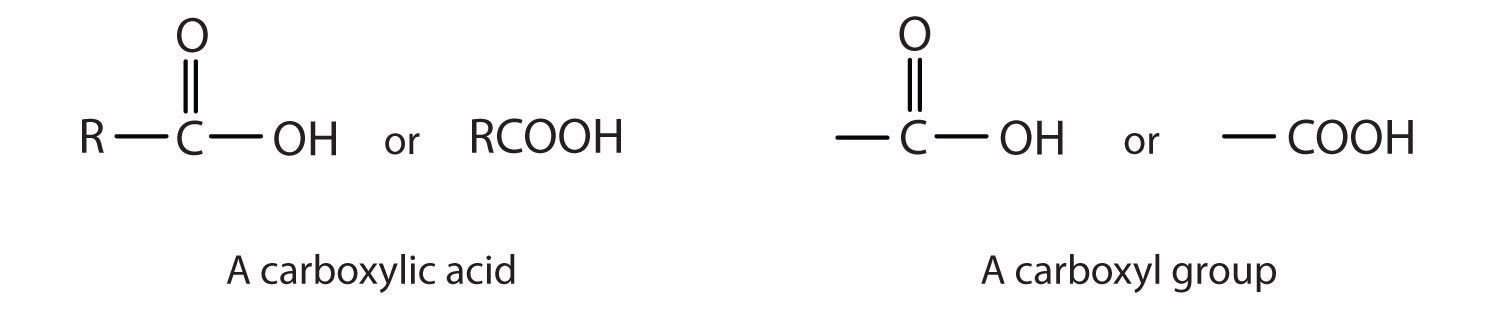
Esters are represented by the formula RCOOR’, where R and R’ are hydrocarbon groups. The ester, which is organic compound derived from a carboxylic acid and an alcohol in which the OH of the acid is replaced by an OR group, looks somewhat like an ether and also somewhat like a carboxylic acid. Even so, compounds in this group react neither like carboxylic acids nor like ethers; they make up a distinctive family. Unlike ethers, esters have a carbonyl group. Unlike carboxylic acids, esters have no acidic hydrogen atom; they have a hydrocarbon group in its place.
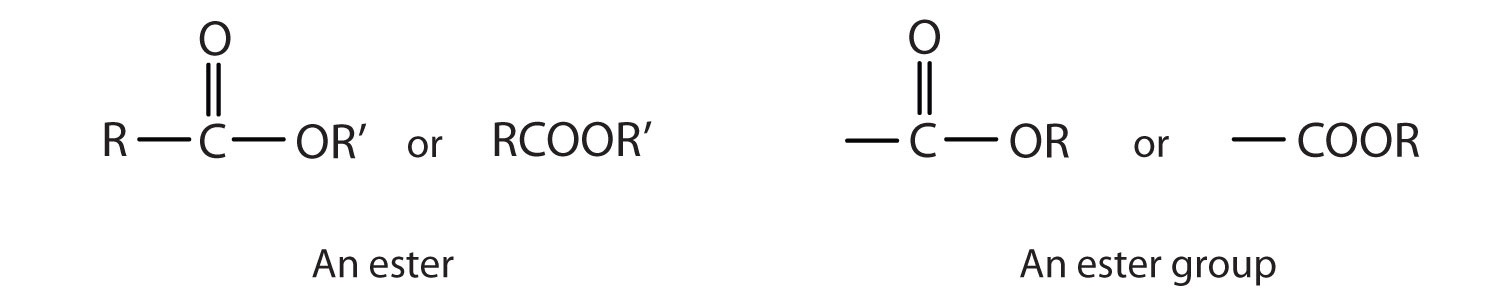
An amine is a compound derived from ammonia (NH3); it has one, two, or all three of the hydrogen atoms of NH3 replaced by an alkyl (or an aryl) group. Like NH3, amines are weak bases. The functional group of an amine is a nitrogen atom with a lone pair of electrons and with one, two, or three alkyl or aryl groups attached.
The amide functional group has a carbonyl group joined to a nitrogen atom from ammonia or an amine. The properties of the amide functional group differ from those of the simple carbonyl group, NH3, and amines.
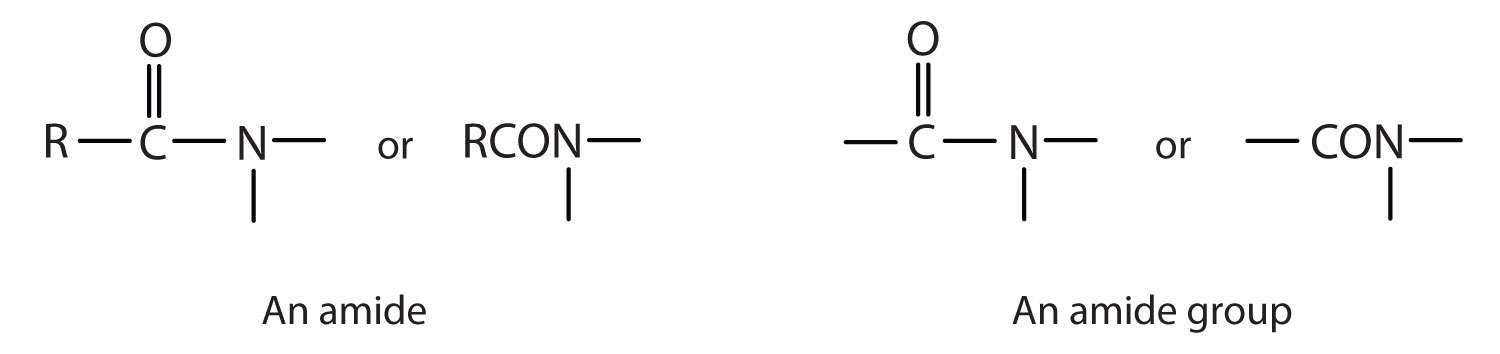
Esters and amides are considered to be derivatives of carboxylic acids because the OH in the carboxyl group is replaced with another group. These functional groups are listed in Table 4.1 "Organic Acids, Bases, and Acid Derivatives", along with an example (identified by common and International Union of Pure and Applied Chemistry [IUPAC] names) for each type of compound.
Most familiar carboxylic acids have an even number of carbon atoms. As we shall see in Chapter 7 "Lipids", these acids—called fatty acids—are synthesized in nature by adding two carbon atoms at a time.
Table 4.1 Organic Acids, Bases, and Acid Derivatives
| Family | Functional Group | Example | Common Name | IUPAC Name |
|---|---|---|---|---|
| carboxylic acid |
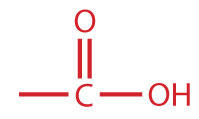 |
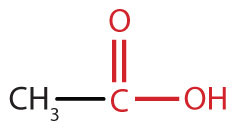 |
acetic acid | ethanoic acid |
| amine |
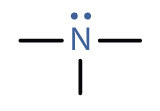 |
CH3NH2 | methylamine | methanamine (aminomethane) |
| amide |
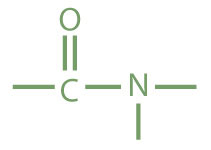 |
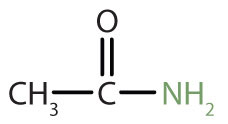 |
acetamide | ethanamide |
| ester |
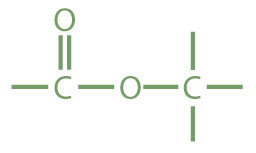 |
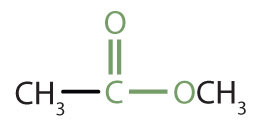 |
methyl acetate | methyl ethanoate |
How is the carboxyl group related to the carbonyl group and the OH group?
How is the amide group related to the carboxyl group and amines?
The carboxyl group has a carbonyl group joined to an OH group.
The amide group has a carboxyl group joined to an amino group.
1. Draw the functional group in each class of compounds.
a. aldehydes
b. esters
c. carboxylic acids
2. How are the functional groups in Exercise 1 alike and different?
3. Draw the functional group in each class of compounds.
a. amides
b. ketones
c. ethers
4. How are the functional groups in Exercise 2 alike and different?
1.
a.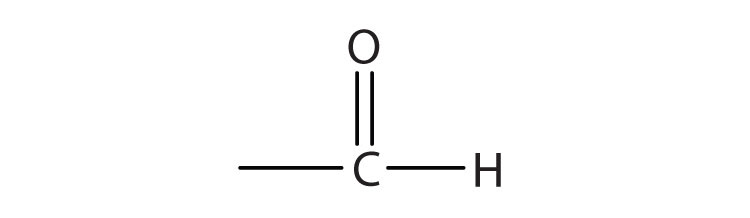
c.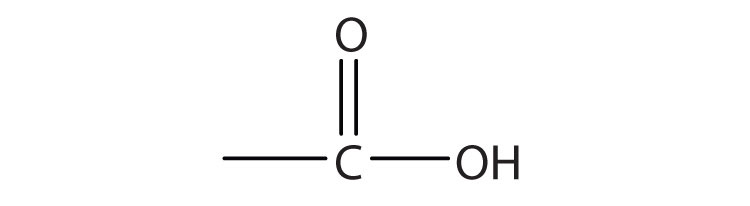
3.
a.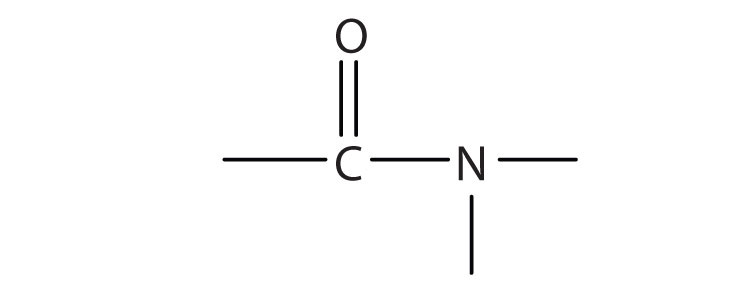
b.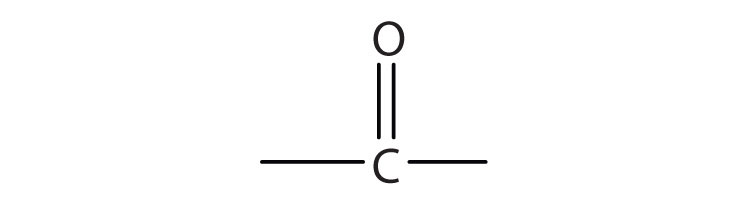
c.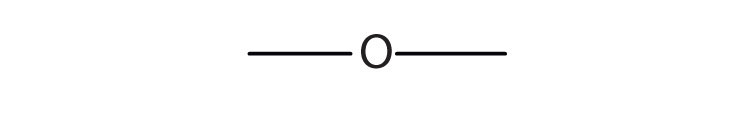
Carboxylic acids occur widely in nature, often combined with alcohols or other functional groups, as in fats, oils, and waxes. They are components of many foods, medicines, and household products. Not surprisingly, many of them are best known by common names based on Latin and Greek words that describe their source. What you learn in this chapter about the chemistry of carboxylic acids will help you understand biochemistry (Chapter 6 "Carbohydrates" through Chapter 11 "Metabolic Pathways and Energy Production").
The simplest carboxylic acid, formic acid (HCOOH), was first obtained by the distillation of ants (Latin formica, meaning “ant”). The bites of some ants inject formic acid, and the stings of wasps and bees contain formic acid (as well as other poisonous materials).
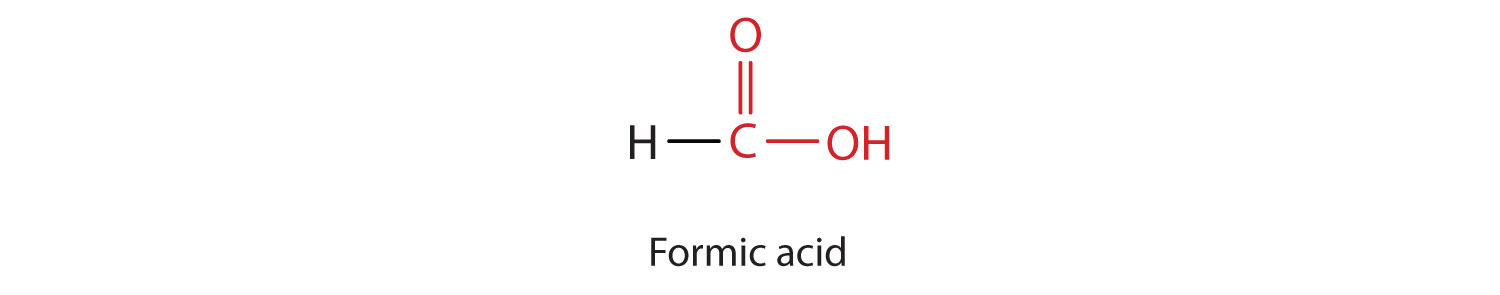
The next higher homolog is acetic acid, which is made by fermenting cider and honey in the presence of oxygen. This fermentation produces vinegar, a solution containing 4%–10% acetic acid, plus a number of other compounds that add to its flavor. Acetic acid is probably the most familiar weak acid used in educational and industrial chemistry laboratories.
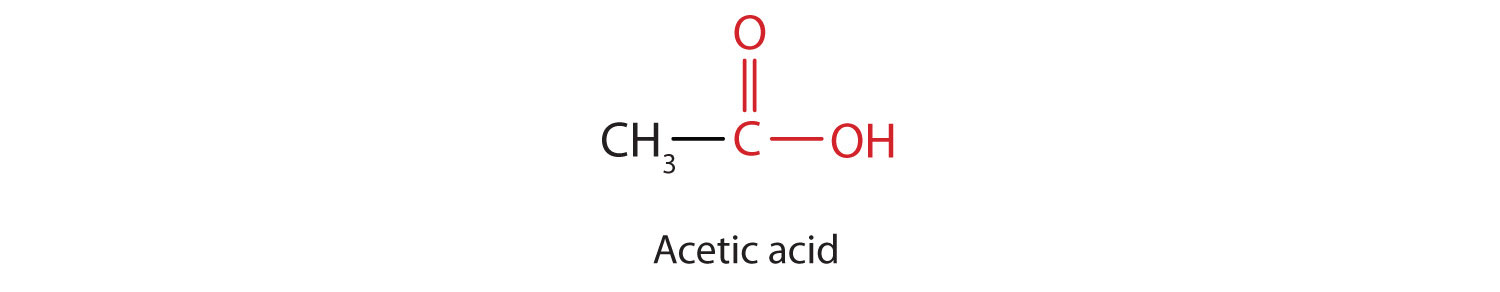
Pure acetic acid solidifies at 16.6°C, only slightly below normal room temperature. In the poorly heated laboratories of the late 19th and early 20th centuries in northern North America and Europe, acetic acid often “froze” on the storage shelf. For that reason, pure acetic acid (sometimes called concentrated acetic acid) came to be known as glacial acetic acid, a name that survives to this day.
The third homolog, propionic acid (CH3CH2COOH), is seldom encountered in everyday life. The fourth homolog, butyric acid (CH3CH2CH2COOH), is one of the most foul-smelling substances imaginable. It is found in rancid butter and is one of the ingredients of body odor. By recognizing extremely small amounts of this and other chemicals, bloodhounds are able to track fugitives. Models of the first four carboxylic acids are shown in Figure 4.1 "Ball-and-Stick Models of Carboxylic Acids".
Figure 4.1 Ball-and-Stick Models of Carboxylic Acids
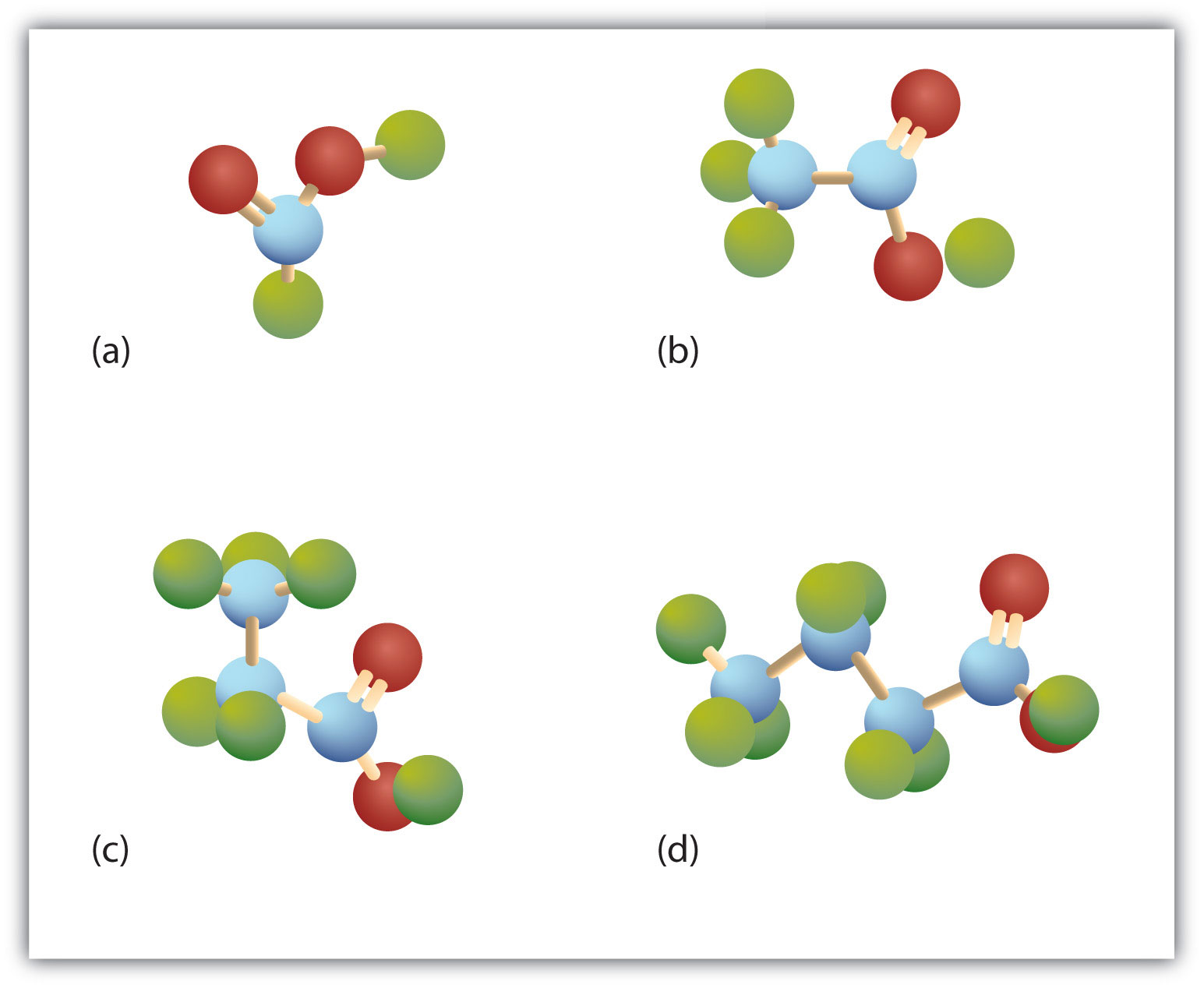
Carboxylic acids feature a carbon atom doubly bonded to an oxygen atom and also joined to an OH group. The four acids illustrated here are formic acid (a), acetic acid (b), propionic acid (c), and butyric acid (d).
The acid with the carboxyl group attached directly to a benzene ring is called benzoic acid (C6H5COOH).
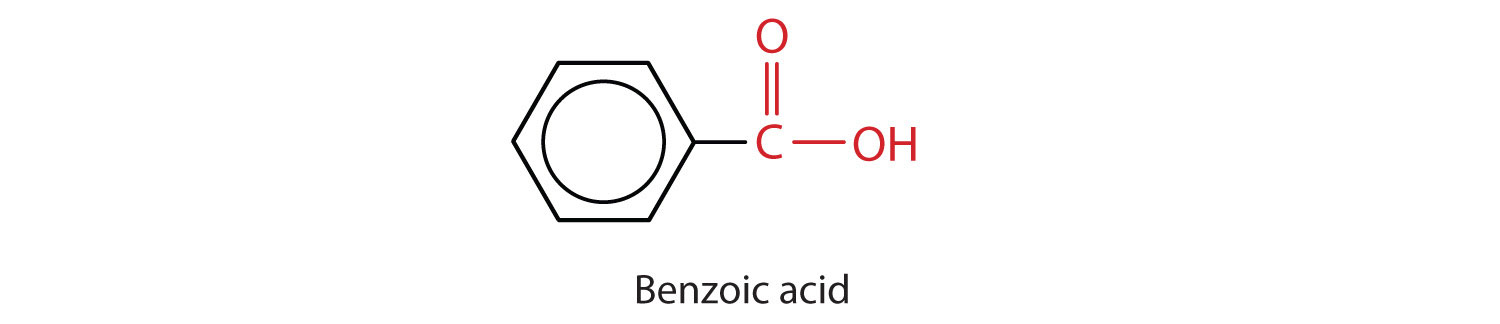
The common names of carboxylic acids use Greek letters (α, β, γ, δ, and so forth), not numbers, to designate the position of substituent groups in acids. These letters refer to the position of the carbon atom in relation to the carboxyl carbon atom.
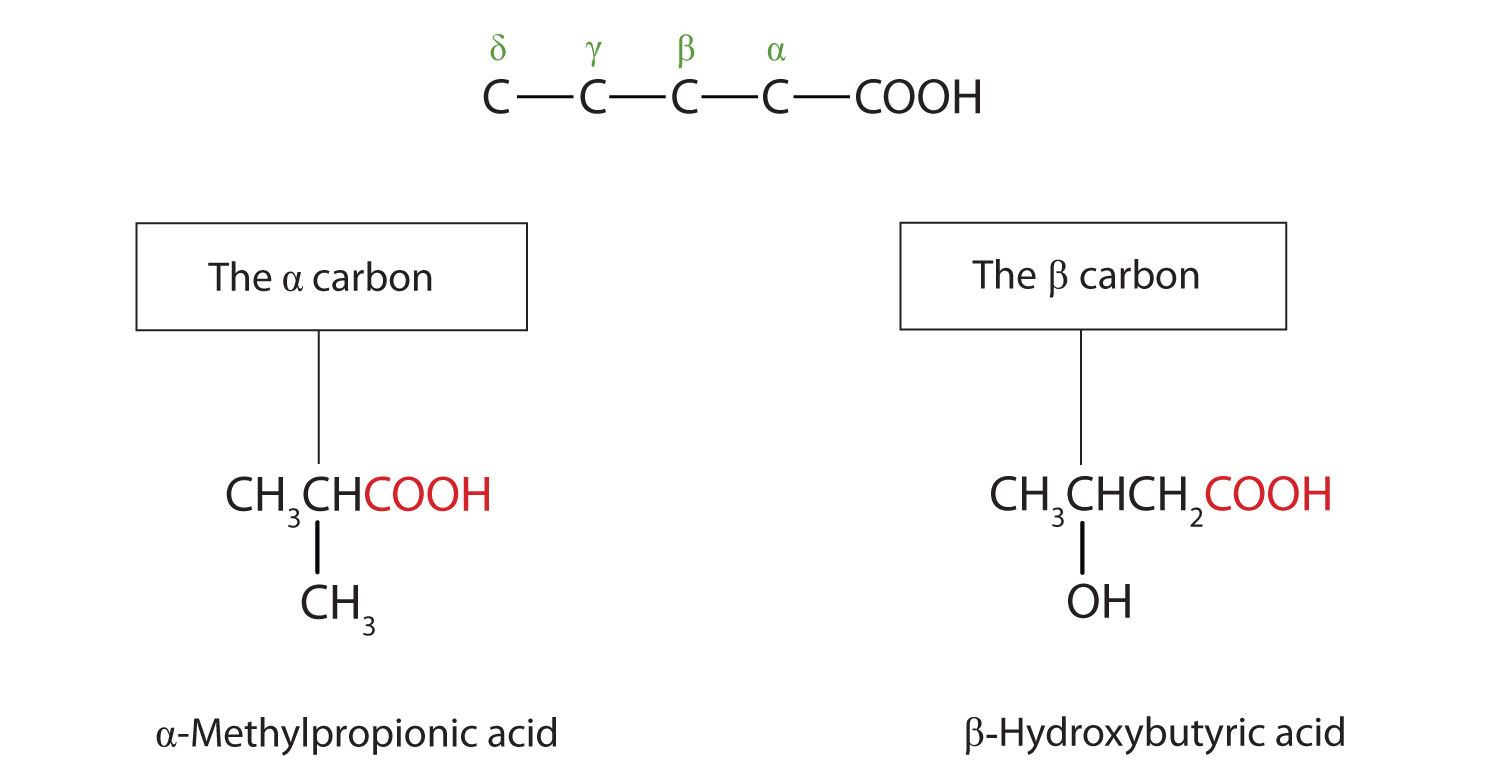
In the nomenclature system of the International Union of Pure and Applied Chemistry (IUPAC), the parent hydrocarbon is the one that corresponds to the longest continuous chain (LCC) containing the carboxyl group. The -e ending of the parent alkane is replaced by the suffix -oic and the word acid. For example, the carboxylic acid derived from pentane is pentanoic acid (CH3CH2CH2CH2COOH). As with aldehydes, the carboxyl carbon atom is counted first; numbers are used to indicate any substituted carbon atoms in the parent chain.
Greek letters are used with common names; numbers are used with IUPAC names.
Give the common and IUPAC names for each compound.
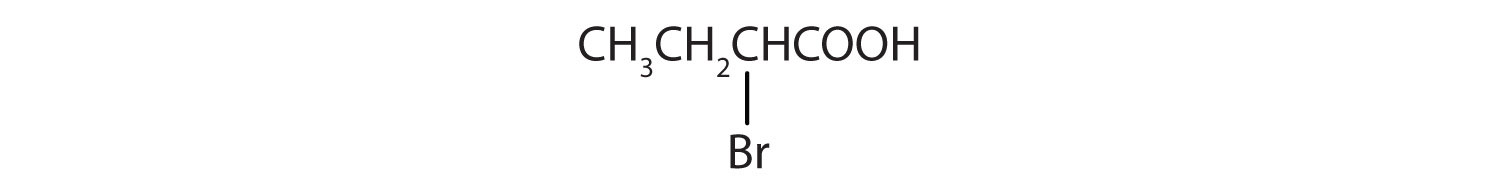
Solution
The LCC contains four carbon atoms; the compound is therefore named as a substituted butyric (or butanoic) acid.
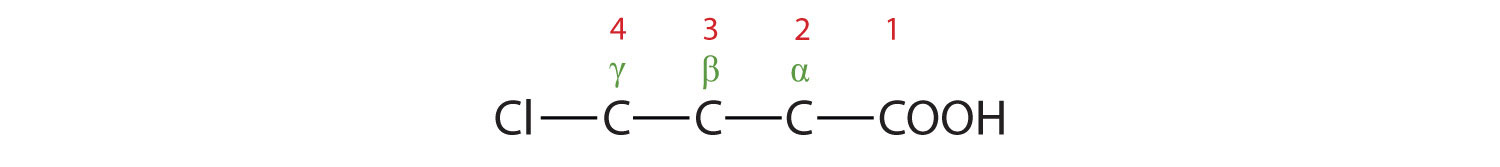
The chlorine atom is attached to the γ-carbon in the common system or C4 in the IUPAC system. The compound is γ-chlorobutyric acid or 2-bromobutanoic acid.
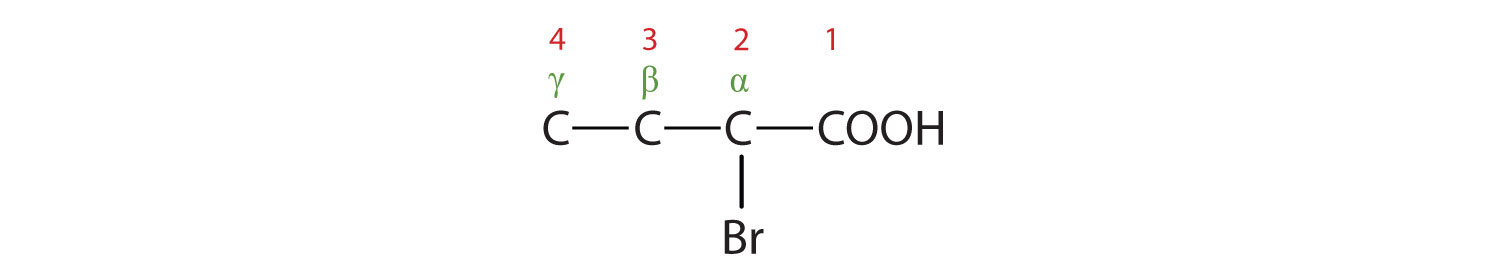
The bromine (Br) atom is at the α-carbon in the common system or C2 in the IUPAC system. The compound is α-bromobutyric acid or 4-chlorobutanoic acid.
Give the IUPAC name for each compound.
ClCH2CH2CH2CH2COOH
(CH3)2CHCH2CHBrCOOH
Write the condensed structural formula for β-chloropropionic acid.
Solution
Propionic acid has three carbon atoms: C–C–COOH. Attach a chlorine (Cl) atom to the parent chain at the beta carbon atom, the second one from the carboxyl group: Cl–C–C–COOH. Then add enough hydrogen atoms to give each carbon atom four bonds: ClCH2CH2COOH.
Write the condensed structural formula for 4-bromo-5-methylhexanoic acid.
What is the IUPAC name for the straight-chain carboxylic acid with six carbon atoms?
The straight-chain aldehyde with five carbon atoms has the common name valeraldehyde. What is the common name of the corresponding straight-chain carboxylic acid?
hexanoic acid
valeric acid
1. Draw the structure for each compound.
a. heptanoic acid
b. 3-methylbutanoic acid
c. 2,3-dibromobenzoic acid
d. β-hydroxybutyric acid
2. Draw the structure for each compound.
a. o-nitrobenzoic acid
b. p-chlorobenzoic acid
c. 3-chloropentanoic acid
d. α-chloropropionic acid
3. Name each compound with either the IUPAC name, the common name, or both.
a. (CH3)2CHCH2COOH
b. (CH3)3CCH(CH3)CH2COOH
c. CH2OHCH2CH2COOH
4. Name each compound with its IUPAC name.
a. CH3(CH2)8COOH
b. (CH3)2CHCCl2CH2CH2COOH
c. CH3CHOHCH(CH2CH3)CHICOOH
1.
a. CH3CH2CH2CH2CH2CH2COOH
b.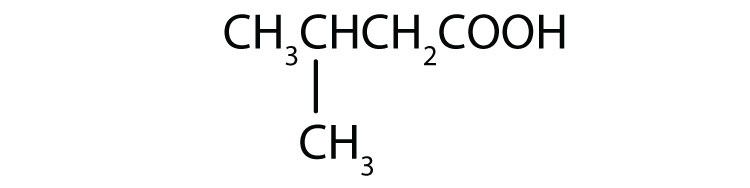
c.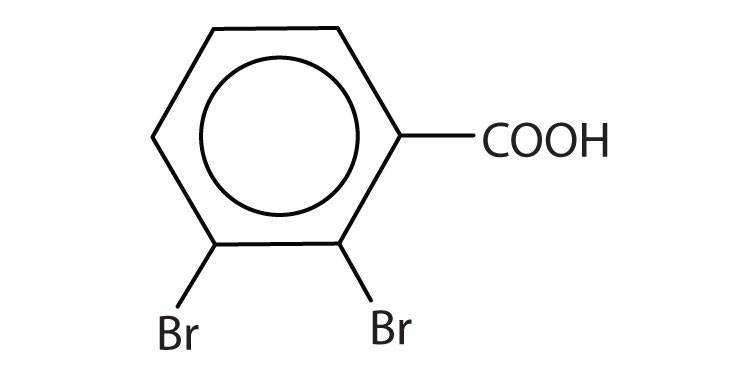
d.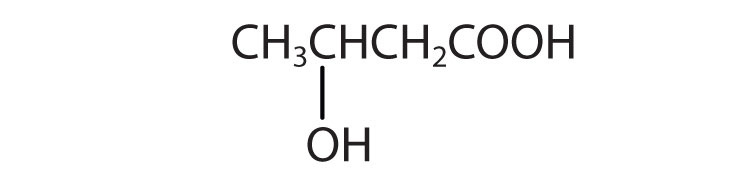
3.
a. 3-methylbutanoic acid; β-methylbutyric acid
b. 3,4,4-trimethylpentanoic acid
c. 4-hydroxybutanoic acid; γ- hydroxybutyric acid
As we noted in Chapter 3 "Aldehydes, Ketones", the oxidation of aldehydes or primary alcohols forms carboxylic acids:
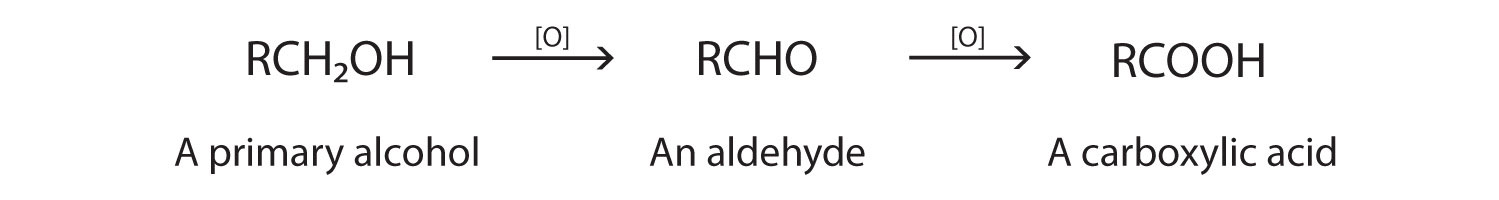
In the presence of an oxidizing agent, ethanol is oxidized to acetaldehyde, which is then oxidized to acetic acid.
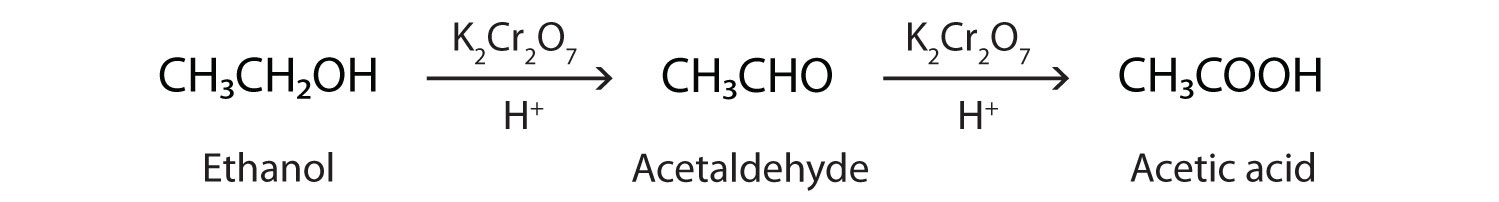
This process also occurs in the liver, where enzymes catalyze the oxidation of ethanol to acetic acid.

Acetic acid can be further oxidized to carbon dioxide and water.
1. Caproic acid (hexanoic acid) can be prepared in an oxidation reaction from
a. what alcohol?
b. what aldehyde?
2. Give the structures of the aldehyde and the carboxylic acid formed by the oxidation of isobutyl alcohol [(CH3)2CHCH2OH].
1.
a. CH3CH2CH2CH2CH2CH2OH
b. CH3CH2CH2CH2CH2CHO
2.
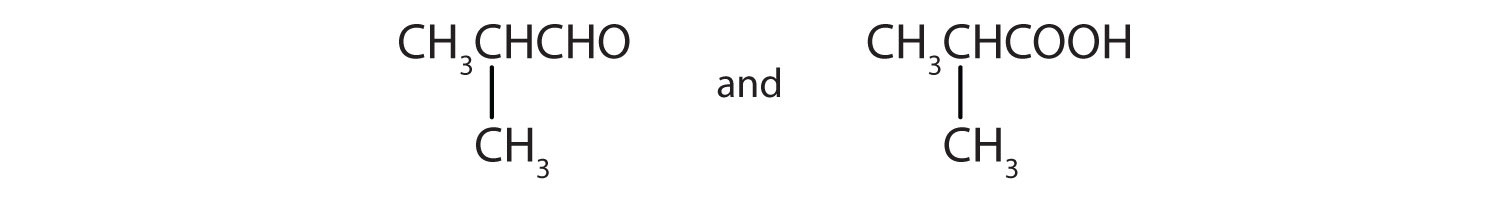
1. Caprylic acid (octanoic acid) can be prepared in an oxidation reaction from
a. what alcohol?
b. what aldehyde?
2. Give the structures of the aldehyde and the carboxylic acid formed by the oxidation of 1,4-butanediol (HOCH2CH2CH2CH2OH).
1.
a. CH3CH2CH2CH2CH2CH2CH2CH2OH
b. CH3CH2CH2CH2CH2CH2CH2CHO
Many carboxylic acids are colorless liquids with disagreeable odors. The carboxylic acids with 5 to 10 carbon atoms all have “goaty” odors (explaining the odor of Limburger cheese). These acids are also produced by the action of skin bacteria on human sebum (skin oils), which accounts for the odor of poorly ventilated locker rooms. The acids with more than 10 carbon atoms are waxlike solids, and their odor diminishes with increasing molar mass and resultant decreasing volatility.
Carboxylic acids exhibit strong hydrogen bonding between molecules. They therefore have high boiling points compared to other substances of comparable molar mass.
The carboxyl group readily engages in hydrogen bonding with water molecules (Figure 4.2 "Hydrogen Bonding between an Acetic Acid Molecule and Water Molecules"). The acids with one to four carbon atoms are completely miscible with water. Solubility decreases as the carbon chain length increases because dipole forces become less important and dispersion forces become more predominant. Hexanoic acid [CH3(CH2)4COOH] is barely soluble in water (about 1.0 g/100 g of water). Palmitic acid [CH3(CH2)14COOH], with its large nonpolar hydrocarbon component, is essentially insoluble in water. The carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether.
Figure 4.2 Hydrogen Bonding between an Acetic Acid Molecule and Water Molecules
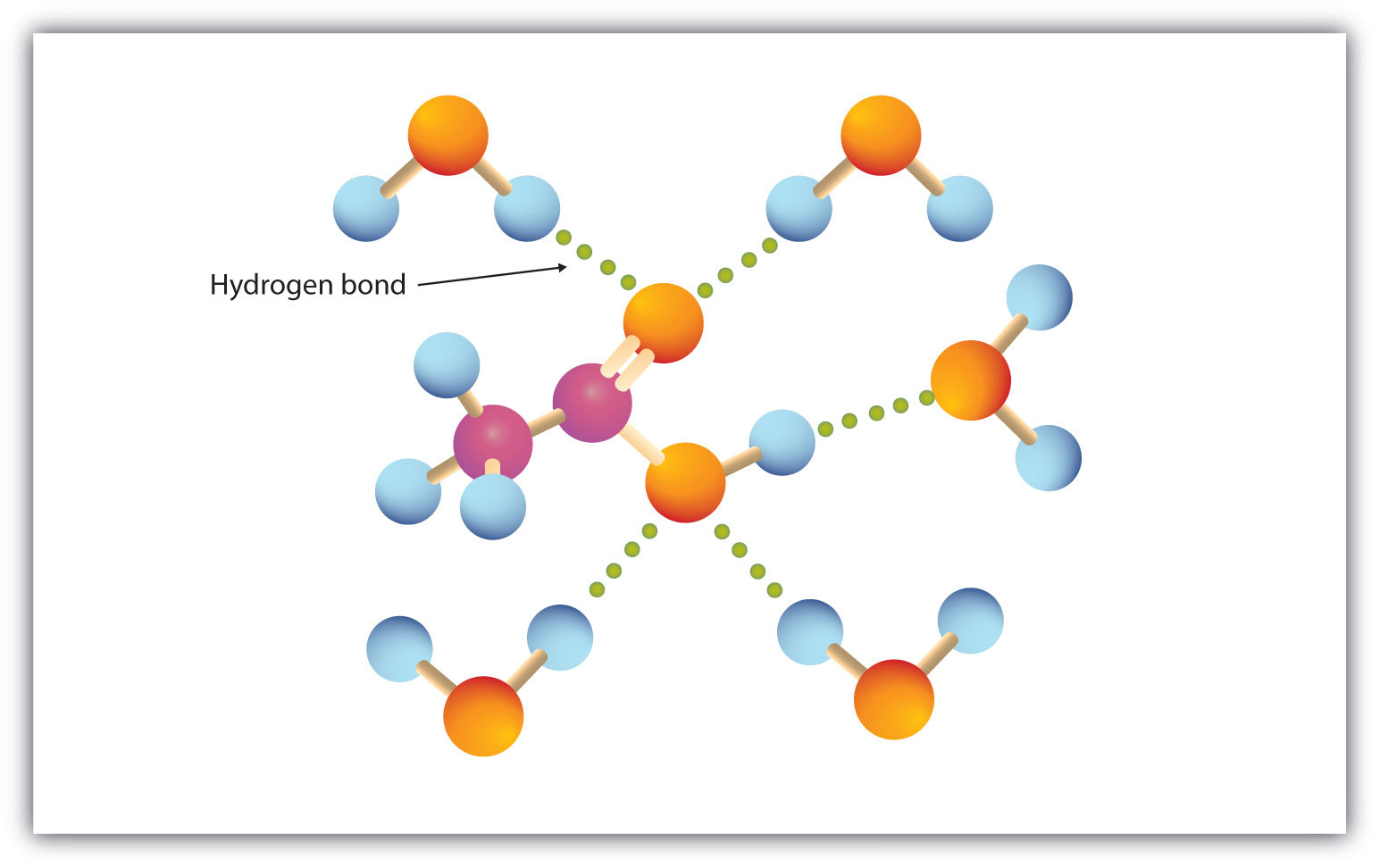
Carboxylic acids of low molar mass are quite soluble in water.
Table 4.2 "Physical Constants of Carboxylic Acids" lists some physical properties for selected carboxylic acids. The first six are homologs. Notice that the boiling points increase with increasing molar mass, but the melting points show no regular pattern.
Table 4.2 Physical Constants of Carboxylic Acids
| Condensed Structural Formula | Name of Acid | Melting Point (°C) | Boiling Point (°C) | Solubility (g/100 g of Water) |
|---|---|---|---|---|
| HCOOH | formic acid | 8 | 100 | miscible |
| CH3COOH | acetic acid | 17 | 118 | miscible |
| CH3CH2COOH | propionic acid | –22 | 141 | miscible |
| CH3(CH2)2COOH | butyric acid | –5 | 163 | miscible |
| CH3(CH2)3COOH | valeric acid | –35 | 187 | 5 |
| CH3(CH2)4COOH | caproic acid | –3 | 205 | 1.1 |
| C6H5COOH | benzoic acid | 122 | 249 | 0.29 |
Which compound has the higher boiling point—butanoic acid (molar mass 88) or 2-pentanone (molar mass 86)? Explain.
Would you expect butyric acid (butanoic acid) to be more or less soluble than 1-butanol in water? Explain.
butyric acid because of hydrogen bonding (There is no intermolecular hydrogen bonding in 2-pentanone.)
more soluble because there is more extensive hydrogen bonding
1. Which compound has the higher boiling point—CH3CH2CH2OCH2CH3 or CH3CH2CH2COOH? Explain.
2. Which compound has the higher boiling point—CH3CH2CH2CH2CH2OH or CH3CH2CH2COOH? Explain.
3. Which compound is more soluble in water—CH3COOH or CH3CH2CH2CH3? Explain.
4. Which compound is more soluble in water—CH3CH2COOH or CH3CH2CH2CH2CH2COOH? Explain.
1. CH3CH2CH2COOH because of hydrogen bonding (There is no intermolecular hydrogen bonding with CH3CH2CH2OCH2CH3.)
3. CH3COOH because it engages in hydrogen bonding with water (There is no intermolecular hydrogen bonding with CH3CH2CH2CH3.)
Water-soluble carboxylic acids ionize slightly in water to form moderately acidic solutions.
Their aqueous solutions exhibit the typical properties of acids, such as changing litmus from blue to red.
The anion formed when a carboxylic acid dissociates is called the carboxylate anion (RCOO−).
Whether soluble in water or not, carboxylic acids react with aqueous solutions of sodium hydroxide (NaOH), sodium carbonate (Na2CO3), and sodium bicarbonate (NaHCO3) to form salts:
RCOOH + NaOH(aq) → RCOO−Na+(aq) + H2O 2RCOOH + Na2CO3(aq) → 2RCOO−Na+(aq) + H2O + CO2(g) RCOOH + NaHCO3(aq) → RCOO−Na+(aq) + H2O + CO2(g)
In these reactions, the carboxylic acids act like inorganic acids: they neutralize basic compounds. With solutions of carbonate (CO3) and bicarbonate (HCO3) ions, they also form carbon dioxide gas.
Carboxylic acid salts are named in the same manner as inorganic salts: the name of the cation is followed by the name of the organic anion. The name of the anion is obtained by dropping the -ic ending of the acid name and replacing it with the suffix -ate. This rule applies whether we are using common names or International Union of Pure and Applied Chemistry (IUPAC) names:

The salts of long-chain carboxylic acids are called soaps. We discuss the chemistry of soaps further in Chapter 7 "Lipids", Section 7.2 "Fats and Oils".
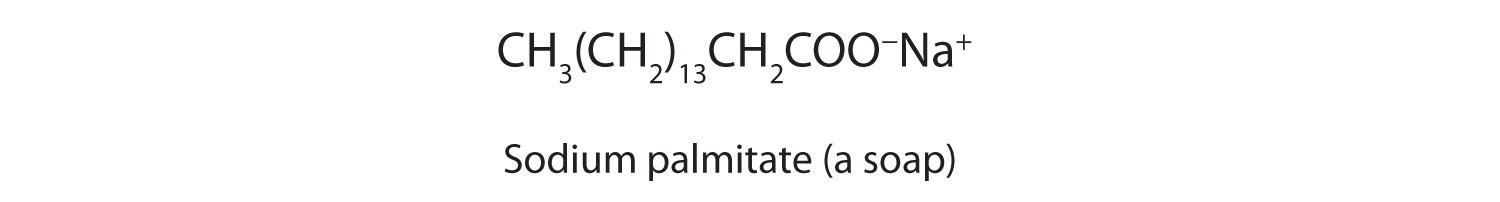
Write an equation for each reaction.
Solution
Propionic acid has three carbon atoms, so its formula is CH2CH2COOH.
Propionic acid ionizes in water to form a propionate ion and a hydronium (H3O+) ion.
CH3CH2COOH(aq) + H2O(ℓ) → CH3CH2COO−(aq) + H3O+(aq)Propionic acid reacts with NaOH(aq) to form sodium propionate and water.
CH3CH2COOH(aq) + NaOH(aq) → CH3CH2COO−Na+(aq) + H2O(ℓ)Write an equation for each reaction.
the ionization of formic acid in water
the ionization of p-chlorobenzoic acid in water
Write an equation for the reaction of decanoic acid with each compound.
Solution
Decanoic acid has 10 carbon atoms. It reacts with NaOH to form a salt and water (H2O).
CH3(CH2)8COOH + NaOH(aq) → CH3(CH2)8COO−Na+(aq) + H2O(ℓ)With NaHCO3, the products are a salt, H2O, and carbon dioxide (CO2).
CH3(CH2)8COOH + NaHCO3(aq) → CH3(CH2)8COO−Na+(aq) + H2O(ℓ) + CO2(g)Write an equation for the reaction of benzoic acid with each compound.
aqueous sodium hydroxide (NaOH)
aqueous sodium bicarbonate (NaHCO3)
Some organic salts are used as preservatives in food products. They prevent spoilage by inhibiting the growth of bacteria and fungi. Calcium and sodium propionate, for example, are added to processed cheese and bakery goods; sodium benzoate is added to cider, jellies, pickles, and syrups; and sodium sorbate and potassium sorbate are added to fruit juices, sauerkraut, soft drinks, and wine. Look for them on ingredient labels the next time you shop for groceries.
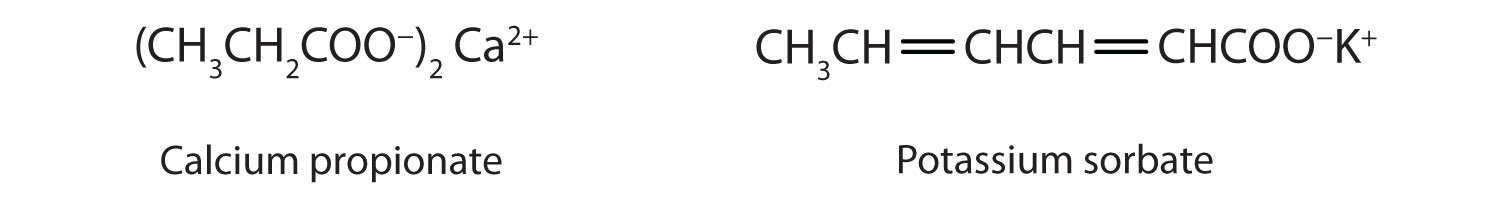
How does the neutralization of a carboxylic acid differ from that of an inorganic acid? How are they similar?
What products are formed when a carboxylic acid is neutralized with a strong base? What additional product is formed when a carboxylic acid is neutralized with a carbonate or a bicarbonate?
Insoluble carboxylic acids often form soluble carboxylate salts. Both form a salt and water.
a carboxylate salt and water; carbon dioxide
1. Write the equation for the ionization of CH3CH2CH2COOH in water.
2. Write the equation for the neutralization of CH3CH2CH2COOH with sodium hydroxide [NaOH(aq)].
3. Write the equation for the reaction of CH3COOH with sodium carbonate [Na2CO3(aq)].
4. Write the equation for the reaction of CH3CH2COOH with sodium bicarbonate [NaHCO3(aq)].
5. Write the equation for the ionization of propionic acid in water.
6. Write the equation for the ionization of γ-chloropentanoic acid in water.
7. Write an equation for the reaction of butyric acid with each compound.
a. aqueous NaOH
b. aqueous NaHCO3
8. Write the condensed structural formula for each compound.
a. potassium acetate
b. calcium propanoate
9. Name each compound.
a. CH3CH2CH2COO−Li+
b. CH3CH2CH2COO−NH4+
1. CH3CH2CH2COOH(aq) + H2O(ℓ) → CH3CH2CH2COO−(aq) + H3O+(aq)
3. 2CH3COOH + Na2CO3(aq) → 2CH3COO−Na+(aq) + H2O(ℓ) + CO2(g)
5. CH3CH2COOH(aq) + H2O(ℓ) → CH3CH2COO−(aq) + H3O+(aq)
7.
a. CH3CH2CH2COOH(aq) + NaOH(aq) → CH3CH2CH2COO−Na+(aq) + H2O(ℓ)
b. CH3(CH2)2COOH + NaHCO3(aq) → CH3(CH2)COO−Na+(aq) + H2O(ℓ) + CO2(g)
9.
a. lithium butyrate (lithium butanoate)
b. ammonium butanoate or ammonium butyrate
Esters have the general formula RCOOR′, where R may be a hydrogen atom, an alkyl group, or an aryl group, and R′ may be an alkyl group or an aryl group but not a hydrogen atom. (If it were hydrogen atom, the compound would be a carboxylic acid.) Figure 4.3 "The Structure of Esters" shows models for two common esters.
Figure 4.3 The Structure of Esters
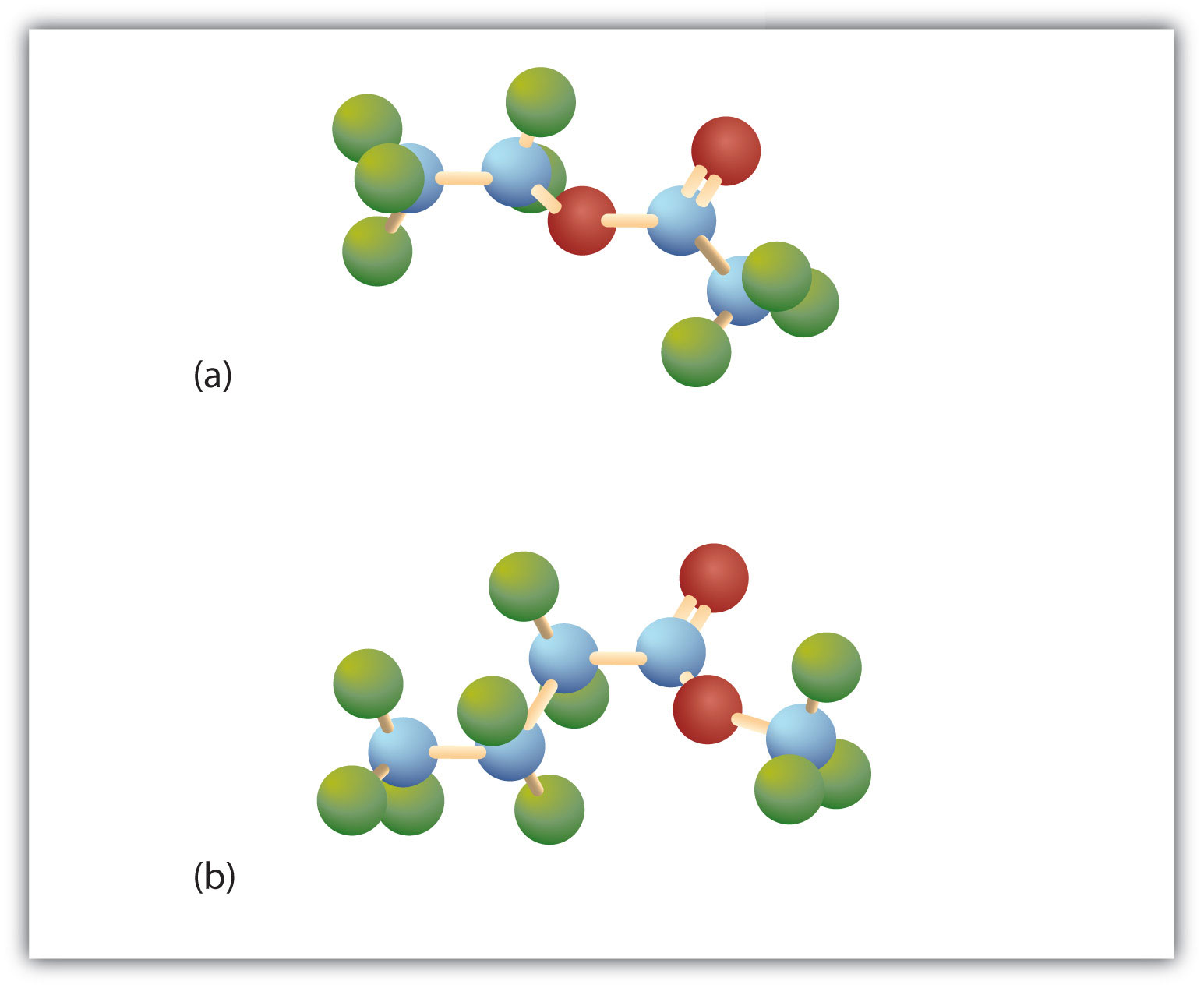
Esters feature a carbon-to-oxygen double bond that is also singly bonded to a second oxygen atom, which is then joined to an alkyl or an aryl group. The esters shown here are ethyl acetate (a) and methyl butyrate (b).
Esters occur widely in nature. Unlike carboxylic acids, esters generally have pleasant odors and are often responsible for the characteristic fragrances of fruits and flowers. Once a flower or fruit has been chemically analyzed, flavor chemists can attempt to duplicate the natural odor or taste. Both natural and synthetic esters are used in perfumes and as flavoring agents.
Fats and vegetable oils are esters of long-chain fatty acids and glycerol. Esters of phosphoric acid are of the utmost importance to life. (For more information about fats/oils and esters, see Chapter 7 "Lipids", Section 7.2 "Fats and Oils", and Section 4.10 "Esters of Phosphoric Acid", respectively.)
Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming salts. The group name of the alkyl or aryl portion is given first and is followed by the name of the acid portion. In both common and International Union of Pure and Applied Chemistry (IUPAC) nomenclature, the -ic ending of the parent acid is replaced by the suffix -ate (Table 4.3 "Nomenclature of Esters").
Table 4.3 Nomenclature of Esters
| Condensed Structural Formula | Common Name | IUPAC Name |
|---|---|---|
| HCOOCH3 | methyl formate | methyl methanoate |
| CH3COOCH3 | methyl acetate | methyl ethanoate |
| CH3COOCH2CH3 | ethyl acetate | ethyl ethanoate |
| CH3CH2COOCH2CH3 | ethyl propionate | ethyl propanoate |
| CH3CH2CH2COOCH(CH3)2 | isopropyl butyrate | isopropyl butanoatey |
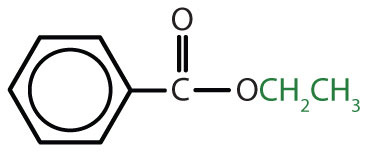 |
ethyl benzoate | ethyl benzoate |
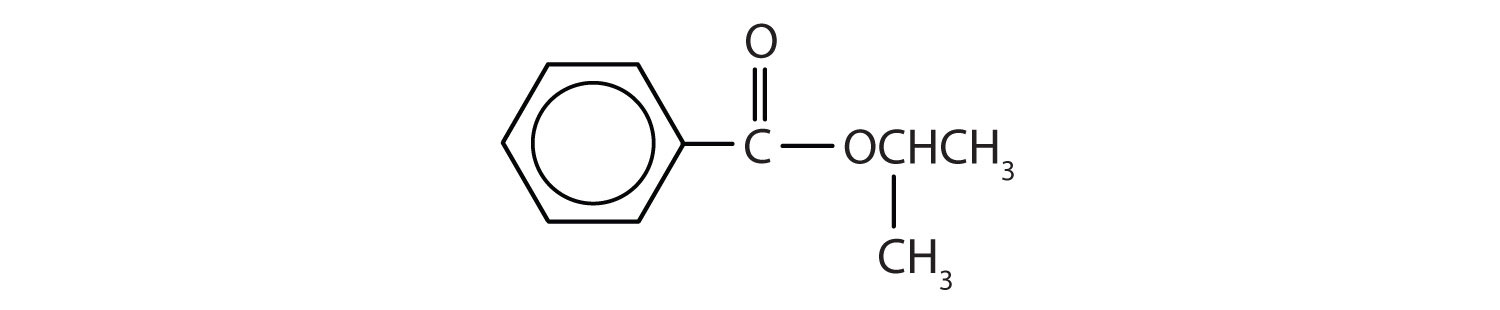
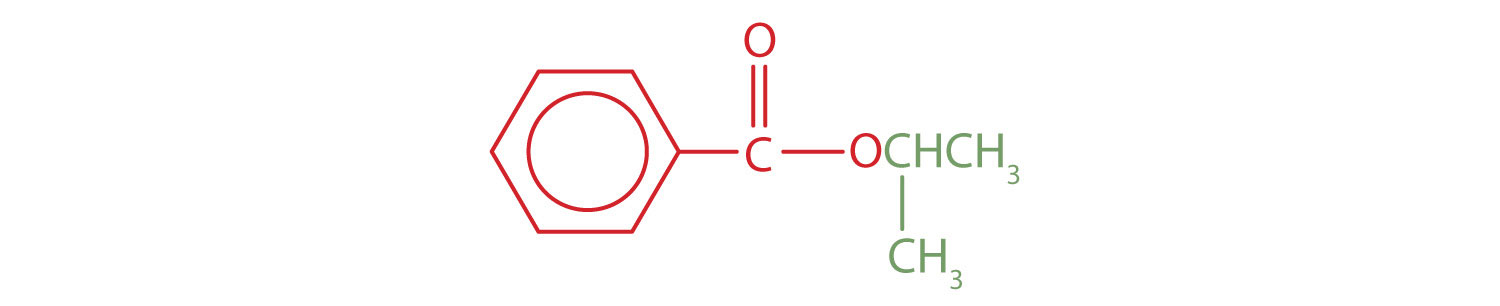
Give the common and IUPAC names for each compound.
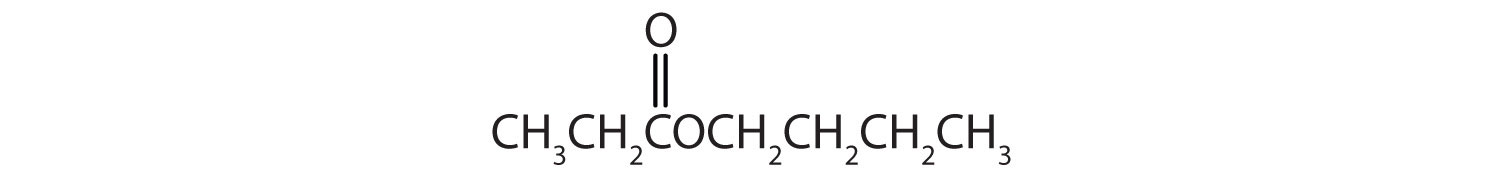
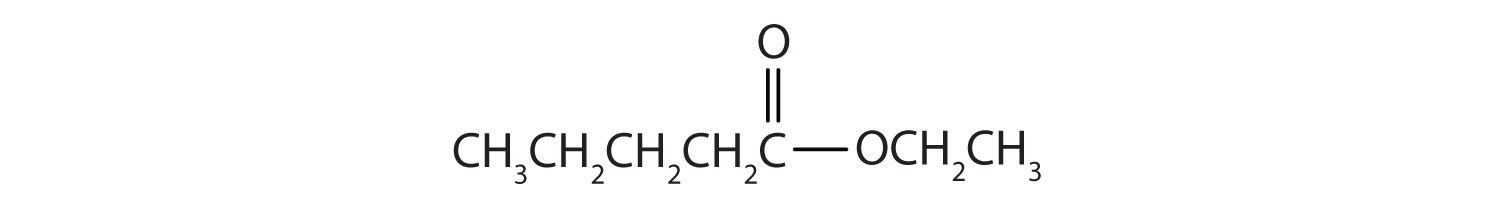
Solution
The alkyl group attached directly to the oxygen atom is a butyl group (in green).
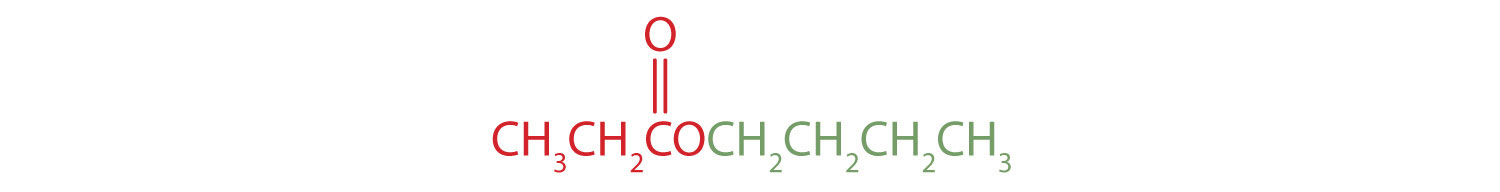
The part of the molecule derived from the carboxylic acid (in red) has three carbon atoms. It is called propionate (common) or propanoate (IUPAC). The ester is therefore butyl propionate or butyl propanoate.
An alkyl group (in green) is attached directly to the oxygen atom by its middle carbon atom; it is an isopropyl group. The part derived from the acid (that is, the benzene ring and the carbonyl group, in red) is benzoate. The ester is therefore isopropyl benzoate (both the common name and the IUPAC name).
Give the common and IUPAC names for each compound.
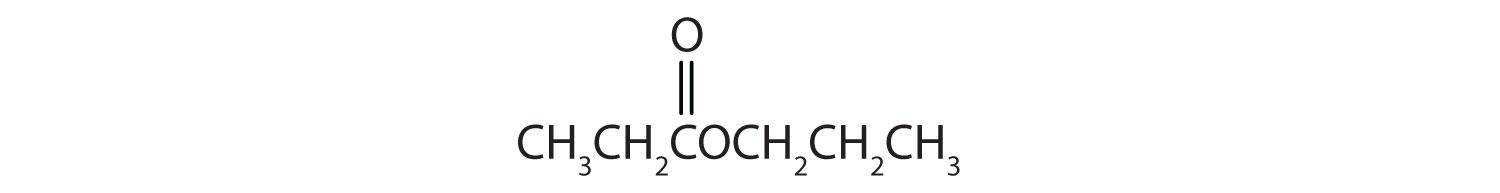
Draw the structure for ethyl pentanoate.
Solution
Start with the portion from the acid. Draw the pentanoate (five carbon atoms) group first; keeping in mind that the last carbon atom is a part of the carboxyl group.
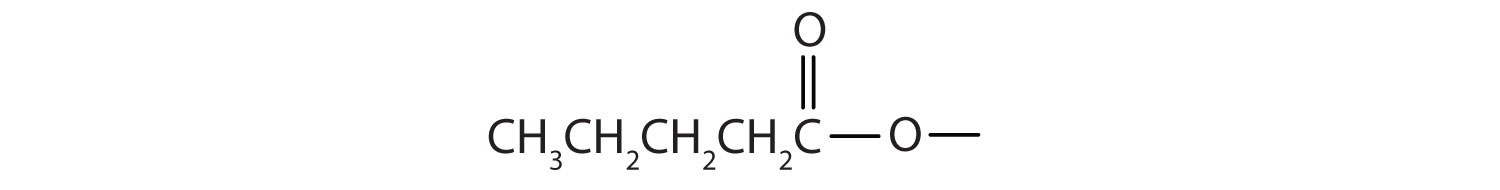
Then attach the ethyl group to the bond that ordinarily holds the hydrogen atom in the carboxyl group.
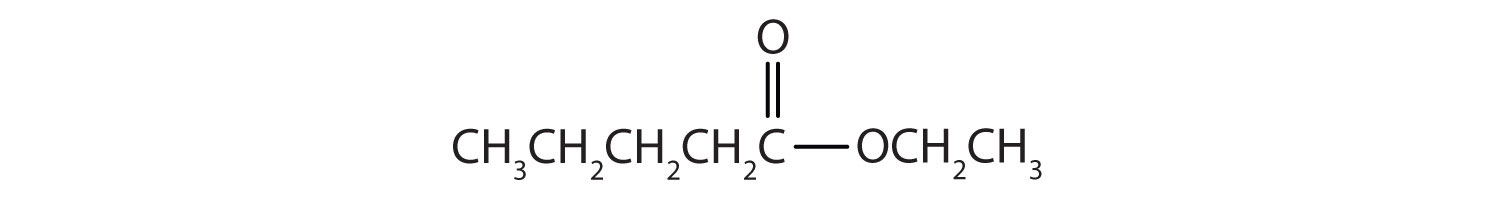
Draw the structure for phenyl pentanoate.
From what carboxylic acid and what alcohol can isopropyl hexanoate be made?
From what carboxylic acid and what alcohol can cyclobutyl butyrate be made?
hexanoic acid and isopropyl alcohol
butyric acid and cyclobutyl alcohol
1. Draw the structure for each compound.
a. methyl acetate
b. ethyl pentanoate
c. phenyl acetate
d. isopropyl propionate
2. Draw the structure for each compound.
a. ethyl hexanoate
b. ethyl benzoate
c. phenyl benzoate
d. ethyl 3-methylhexanoate
3. Name each compound with both the common name and the IUPAC name.
a.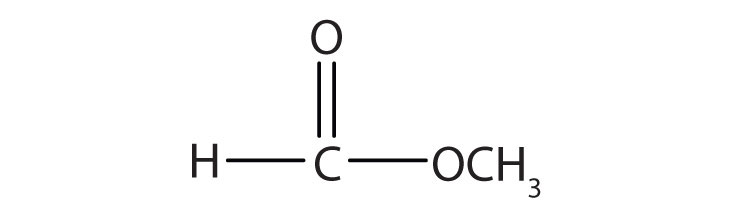
b.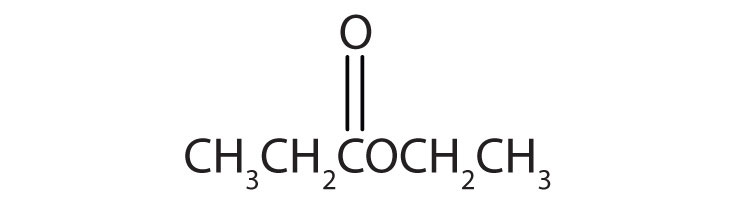
4. Name each compound with both the common name and the IUPAC name.
a. 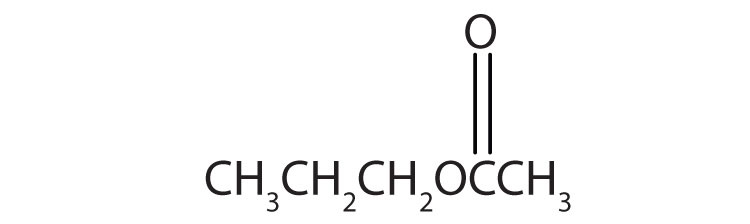
b.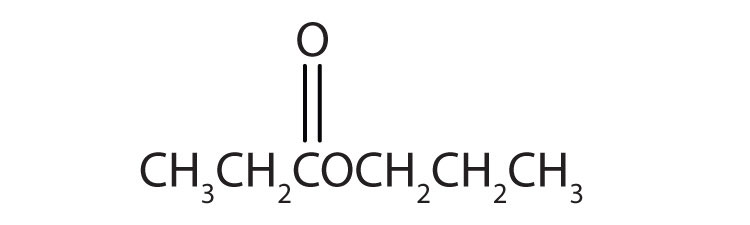
1.
a. 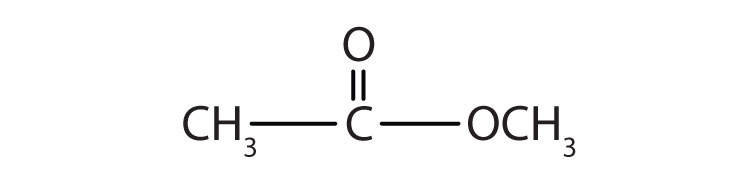
b.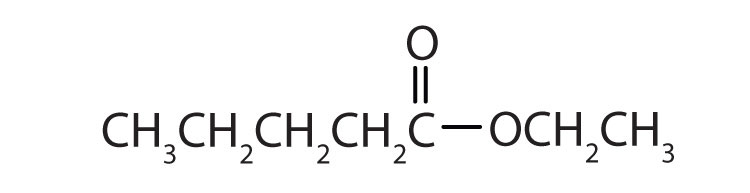
c.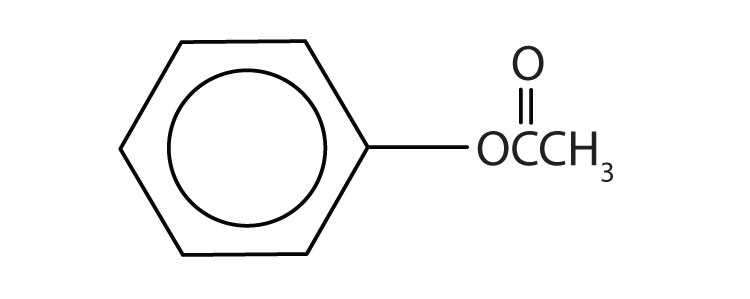
d.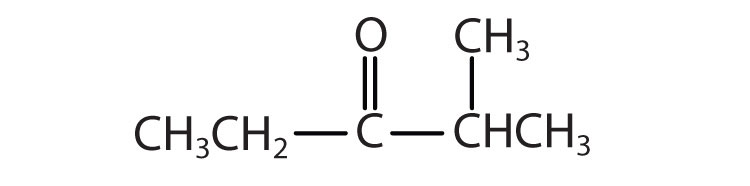
3.
a. methyl formate; methyl methanoate
b. ethyl propionate; ethyl propanoate
Ester molecules are polar but have no hydrogen atom attached directly to an oxygen atom. They are therefore incapable of engaging in intermolecular hydrogen bonding with one another and thus have considerably lower boiling points than their isomeric carboxylic acids counterparts. Because ester molecules can engage in hydrogen bonding with water molecules, however, esters of low molar mass are somewhat soluble in water. Borderline solubility occurs in those molecules that have three to five carbon atoms. Table 4.4 "Physical Properties of Some Esters" lists the physical properties of some common esters.
Esters are common solvents. Ethyl acetate is used to extract organic solutes from aqueous solutions—for example, to remove caffeine from coffee. It also is used to remove nail polish and paint. Cellulose nitrate is dissolved in ethyl acetate and butyl acetate to form lacquers. The solvent evaporates as the lacquer “dries,” leaving a thin film on the surface. High boiling esters are used as softeners (plasticizers) for brittle plastics.
Table 4.4 Physical Properties of Some Esters
| Condensed Structural Formula | Name | Molar Mass | Melting Point (°C) | Boiling Point (°C) | Aroma |
|---|---|---|---|---|---|
| HCOOCH3 | methyl formate | 60 | −99 | 32 | |
| HCOOCH2CH3 | ethyl formate | 74 | −80 | 54 | rum |
| CH3COOCH3 | methyl acetate | 74 | −98 | 57 | |
| CH3COOCH2CH3 | ethyl acetate | 88 | −84 | 77 | |
| CH3CH2CH2COOCH3 | methyl butyrate | 102 | −85 | 102 | apple |
| CH3CH2CH2COOCH2CH3 | ethyl butyrate | 116 | −101 | 121 | pineapple |
| CH3COO(CH2)4CH3 | pentyl acetate | 130 | −71 | 148 | pear |
| CH3COOCH2CH2CH(CH3)2 | isopentyl acetate | 130 | −79 | 142 | banana |
| CH3COOCH2C6H5 | benzyl acetate | 150 | −51 | 215 | jasmine |
| CH3CH2CH2COO(CH2)4CH3 | pentyl butyrate | 158 | −73 | 185 | apricot |
| CH3COO(CH2)7CH3 | octyl acetate | 172 | −39 | 210 | orange |
Which compound has the higher boiling point—CH3CH2CH2CH2OH or CH3COOCH3? Explain.
Which compound is more soluble in water—methyl butyrate or butyric acid? Explain.
CH3CH2CH2CH2OH because there is intermolecular hydrogen bonding (There is no intermolecular hydrogen bonding in CH3COOCH3.)
butyric acid because of hydrogen bonding with water
Which compound has the higher boiling point—CH3CH2CH2COOH or CH3CH2CH2COOCH3? Explain.
Which compound is more soluble in water—methyl acetate or octyl acetate? Explain.
CH3CH2CH2COOH because there is intermolecular hydrogen bonding (There is no intermolecular hydrogen bonding in CH3CH2COOCH3.)
Some esters can be prepared by esterification, a reaction in which a carboxylic acid and an alcohol, heated in the presence of a mineral acid catalyst, form an ester and water:
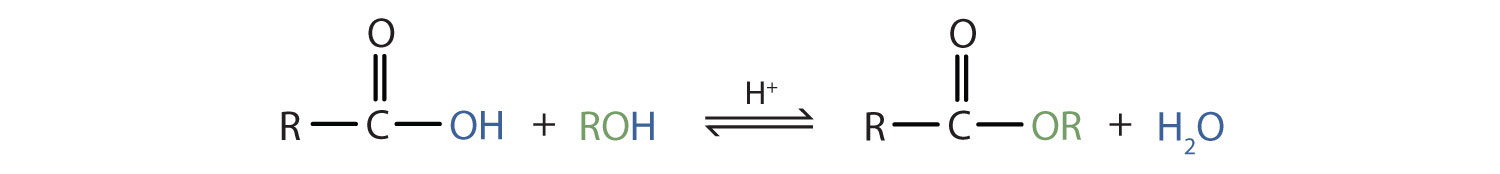
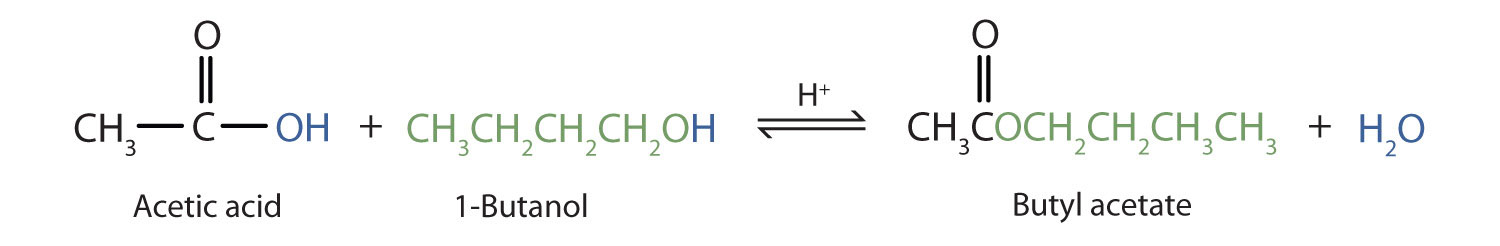
The reaction is reversible. As a specific example of an esterification reaction, butyl acetate can be made from acetic acid and 1-butanol.
A commercially important esterification reaction is condensation polymerization, in which a reaction occurs between a dicarboxylic acid and a dihydric alcohol (diol), with the elimination of water. Such a reaction yields an ester that contains a free (unreacted) carboxyl group at one end and a free alcohol group at the other end. Further condensation reactions then occur, producing polyester polymers.
The most important polyester, polyethylene terephthalate (PET), is made from terephthalic acid and ethylene glycol monomers:
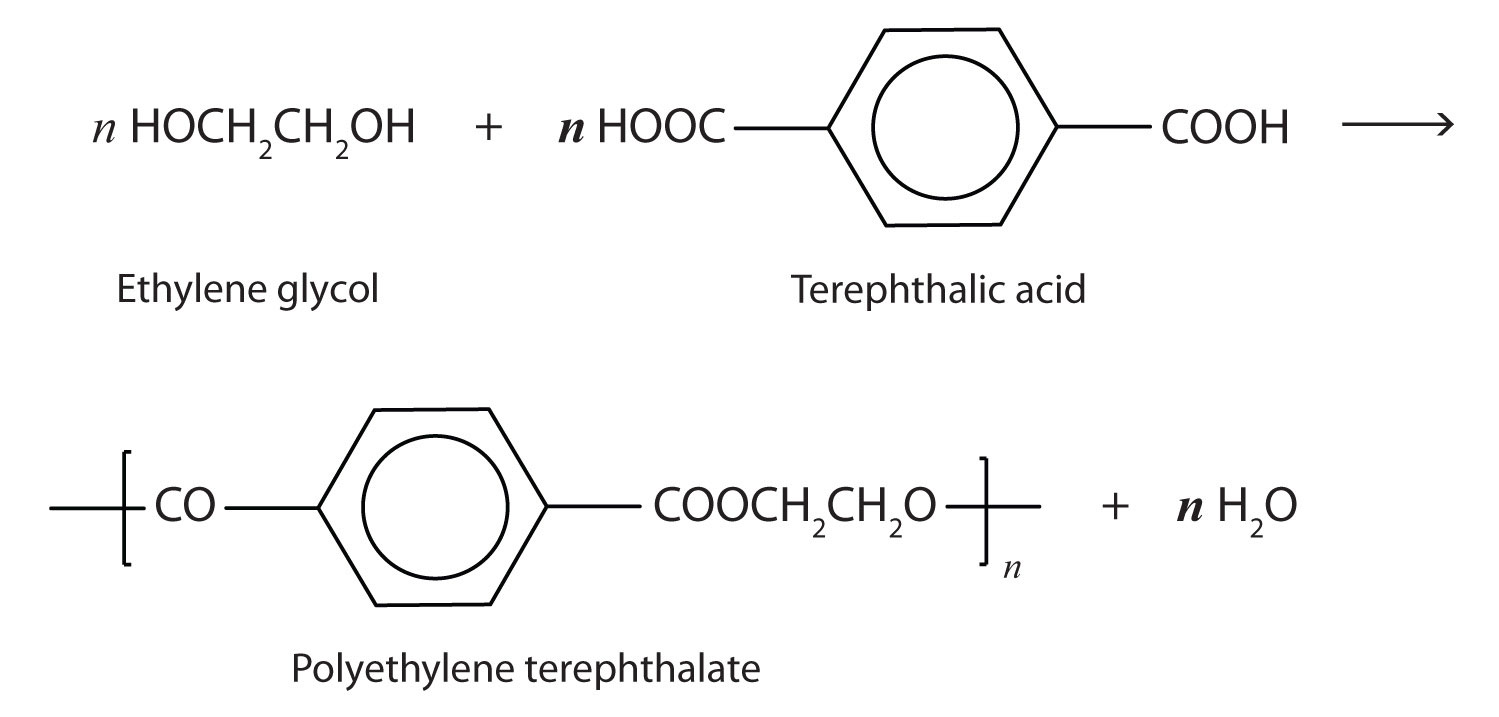
Polyester molecules make excellent fibers and are used in many fabrics. A knitted polyester tube, which is biologically inert, can be used in surgery to repair or replace diseased sections of blood vessels. PET is used to make bottles for soda pop and other beverages. It is also formed into films called Mylar. When magnetically coated, Mylar tape is used in audio- and videocassettes. Synthetic arteries can be made from PET, polytetrafluoroethylene, and other polymers.
From what carboxylic acid and what alcohol can the ester isopropyl nonanoate be made?
From what carboxylic acid and what alcohol can the ester cyclobutyl butyrate be made?
nonanoic acid and isopropyl alcohol
butyric acid and cyclobutyl alcohol
1. Write the equation for the reaction of acetic acid with each compound.
a. ethanol
b. 1-butanol in the presence of a mineral acid catalyst
2. Write the equation for the reaction of benzoic acid with each compound.
a. methanol
b. 1-propanol in the presence of a mineral acid catalyst
1.
a.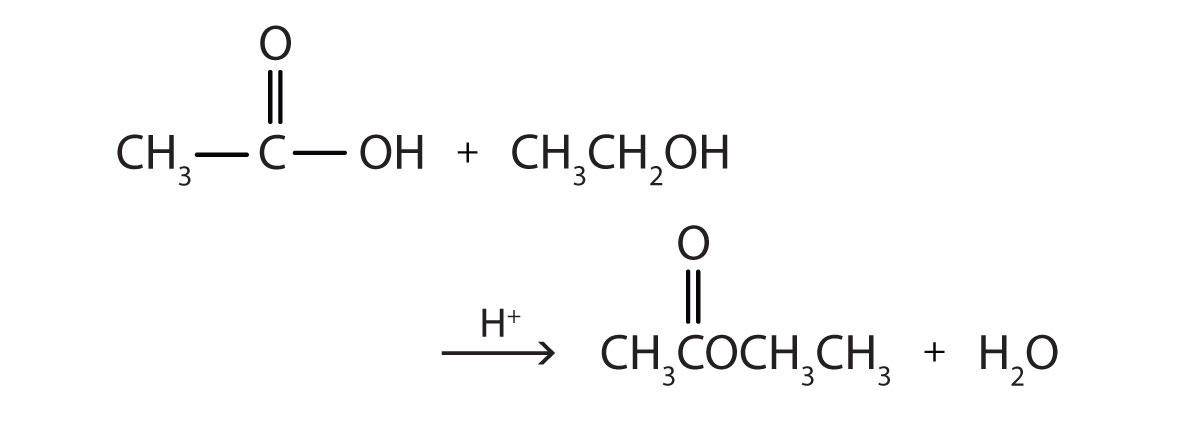
b.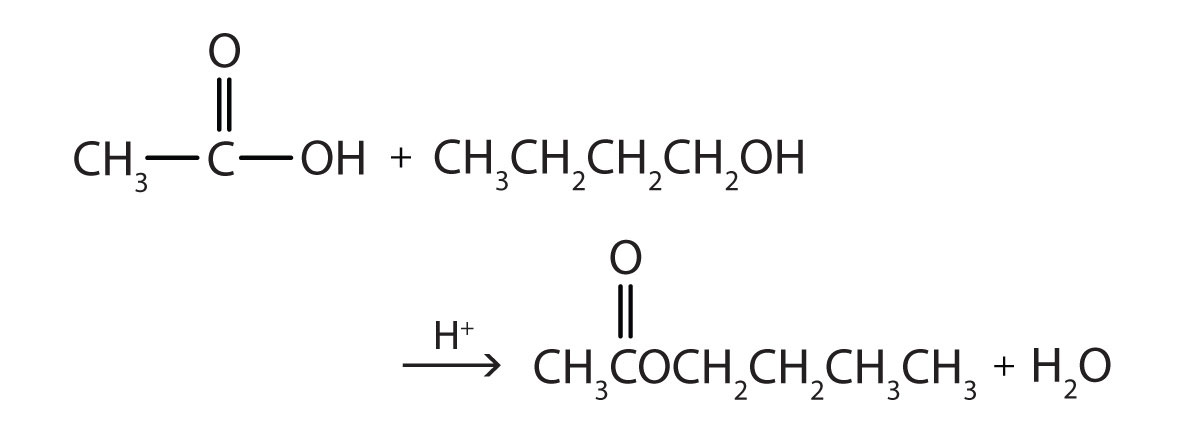
Esters are neutral compounds, unlike the acids from which they are formed. In typical reactions, the alkoxy (OR′) group of an ester is replaced by another group. One such reaction is hydrolysis, literally “splitting with water.” The hydrolysis of esters is catalyzed by either an acid or a base.
Acidic hydrolysis is simply the reverse of esterification. The ester is heated with a large excess of water containing a strong-acid catalyst. Like esterification, the reaction is reversible and does not go to completion.
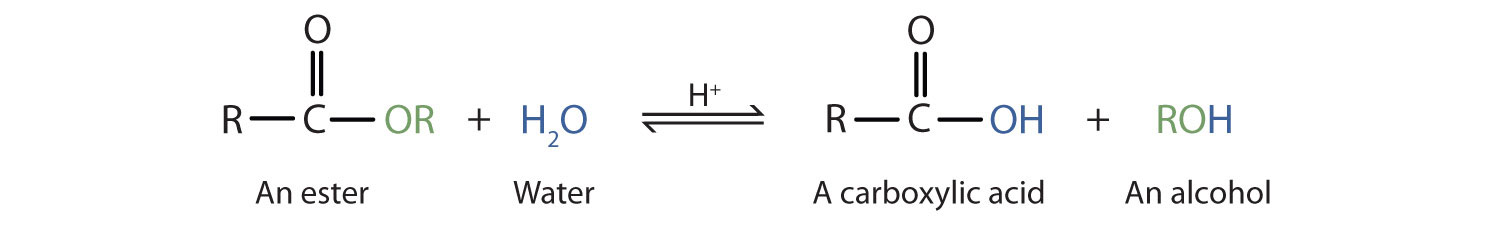
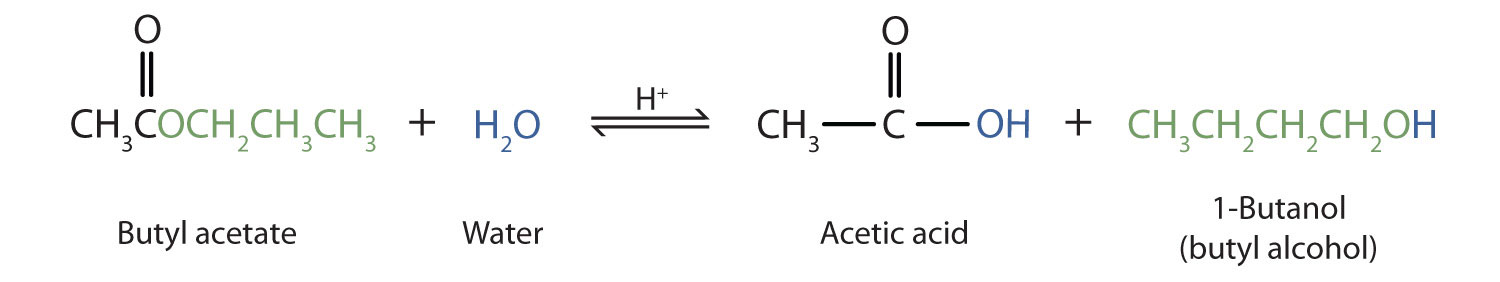
As a specific example, butyl acetate and water react to form acetic acid and 1-butanol. The reaction is reversible and does not go to completion.
Write an equation for the acidic hydrolysis of ethyl butyrate (CH3CH2CH2COOCH2CH3) and name the products.
Solution
Remember that in acidic hydrolysis, water (HOH) splits the ester bond. The H of HOH joins to the oxygen atom in the OR part of the original ester, and the OH of HOH joins to the carbonyl carbon atom:
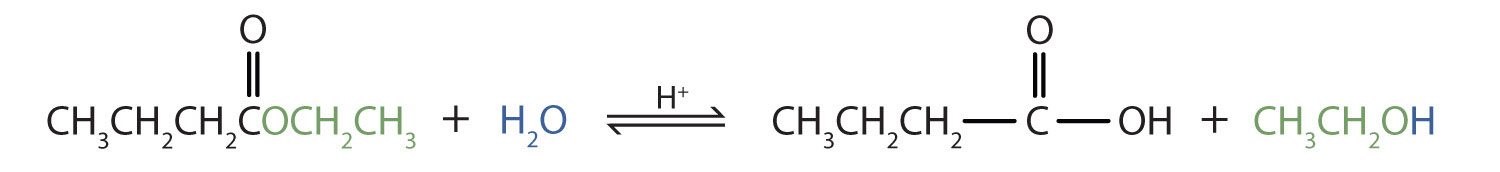
The products are butyric acid (butanoic acid) and ethanol.
Write an equation for the acidic hydrolysis of methyl butanoate and name the products.
When a base (such as sodium hydroxide [NaOH] or potassium hydroxide [KOH]) is used to hydrolyze an ester, the products are a carboxylate salt and an alcohol. Because soaps are prepared by the alkaline hydrolysis of fats and oils, alkaline hydrolysis of esters is called saponification (Latin sapon, meaning “soap,” and facere, meaning “to make”). In a saponification reaction, the base is a reactant, not simply a catalyst. The reaction goes to completion:
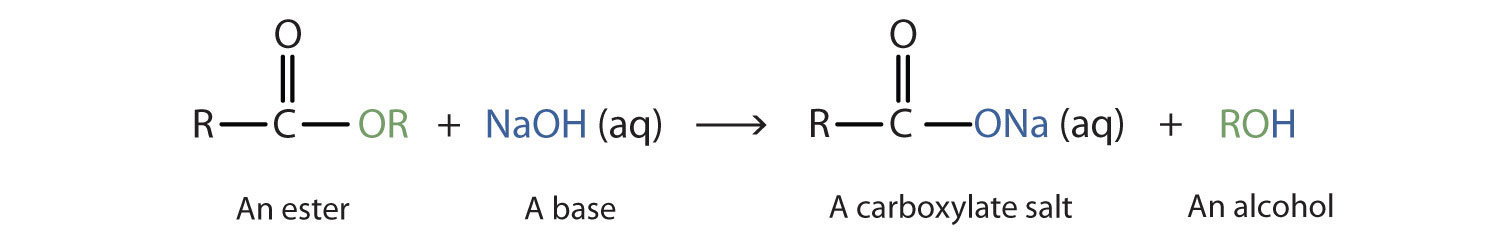
As a specific example, ethyl acetate and NaOH react to form sodium acetate and ethanol:
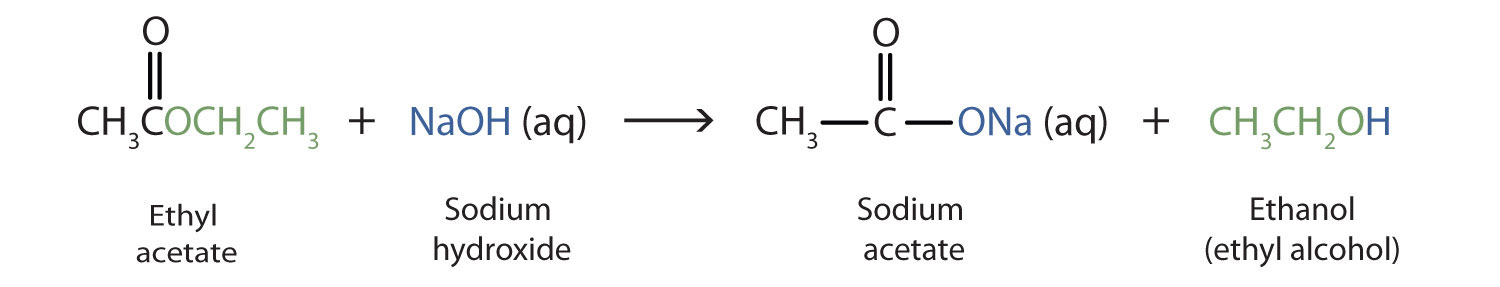
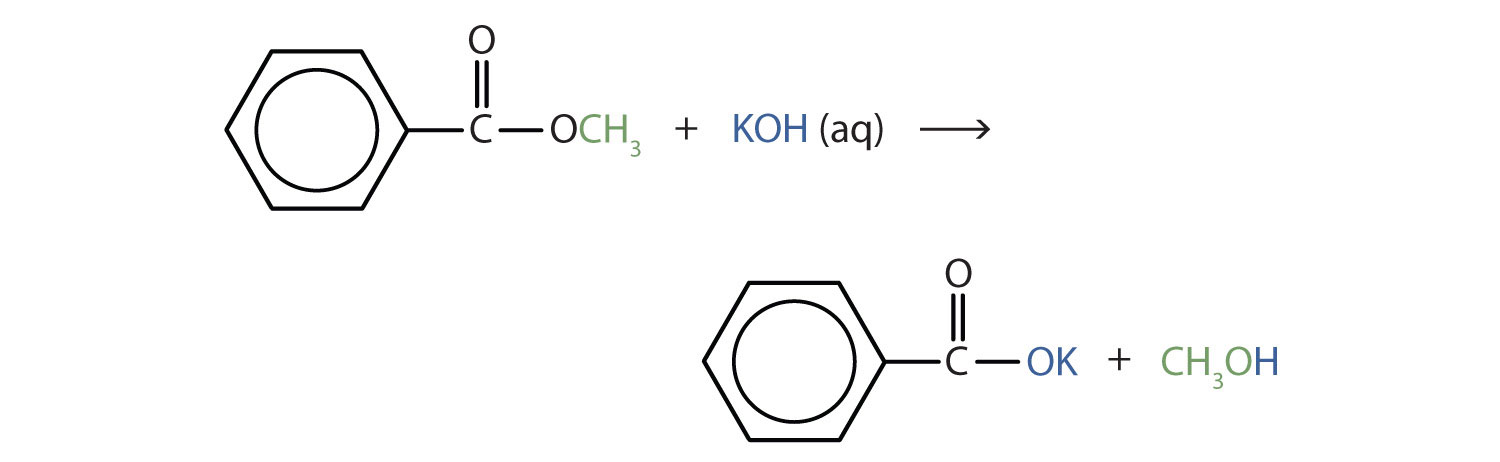
Write an equation for the hydrolysis of methyl benzoate in a potassium hydroxide solution.
Solution
In basic hydrolysis, the molecule of the base splits the ester linkage. The acid portion of the ester ends up as the salt of the acid (in this case, the potassium salt). The alcohol portion of the ester ends up as the free alcohol.
Write the equation for the hydrolysis of ethyl propanoate in a sodium hydroxide solution.
1. How do acidic hydrolysis and basic hydrolysis of an ester differ in terms of
a. products obtained?
b. the extent of reaction?
2. What is saponification?
1.
a. acidic hydrolysis: carboxylic acid + alcohol; basic hydrolysis: carboxylate salt + alcohol
b. basic hydrolysis: completion; acidic hydrolysis: incomplete reaction
2. the basic hydrolysis of an ester
1. Write an equation for the acid-catalyzed hydrolysis of ethyl acetate.
2. Write an equation for the base-catalyzed hydrolysis of ethyl acetate.
3. Complete each equation.
a.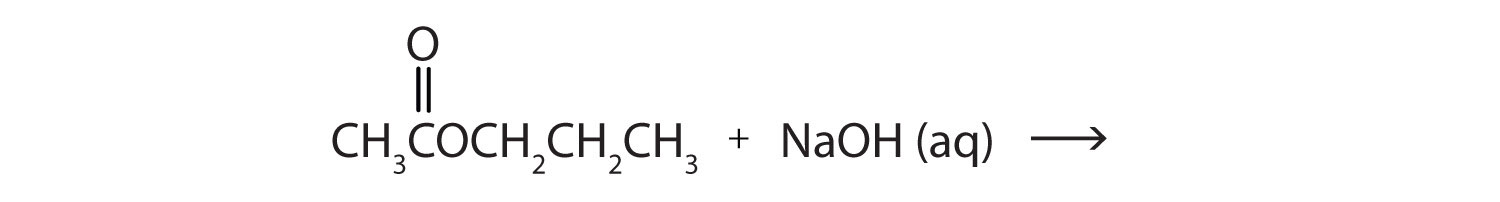
b.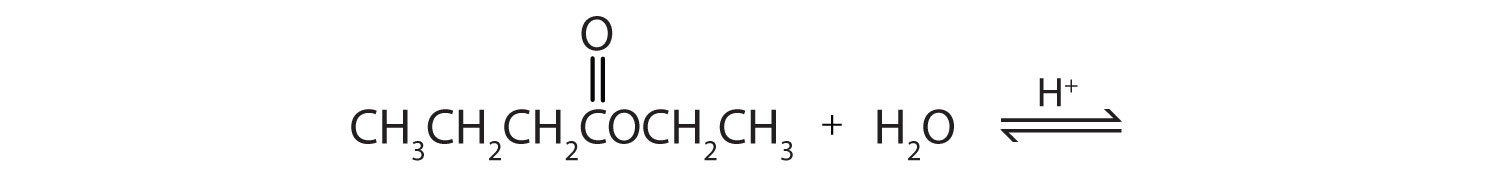
4. Complete each equation.
a. 
b. CH3COOCH(CH3)2 + KOH(aq) →
1. 
3.
a. CH3COONa(aq) + CH3CH2CH2OH
b. CH3CH2CH2COOH + CH3CH2OH
Just as carboxylic acids do, inorganic acids such as nitric acid (HNO3), sulfuric acid (H2SO4), and phosphoric acid (H3PO4) also form esters. The esters of phosphoric acid are especially important in biochemistry. A phosphoric acid molecule can form a monoalkyl, a dialkyl, or a trialkyl ester by reaction with one, two, or three molecules of an alcohol.
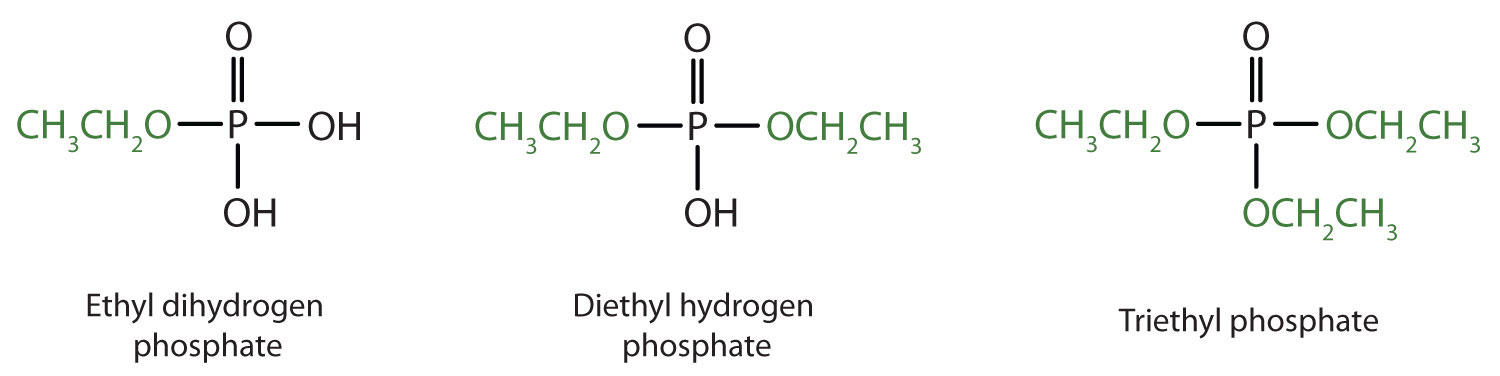
Esters of pyrophosphoric acid and triphosphoric acid are also important in biochemistry.
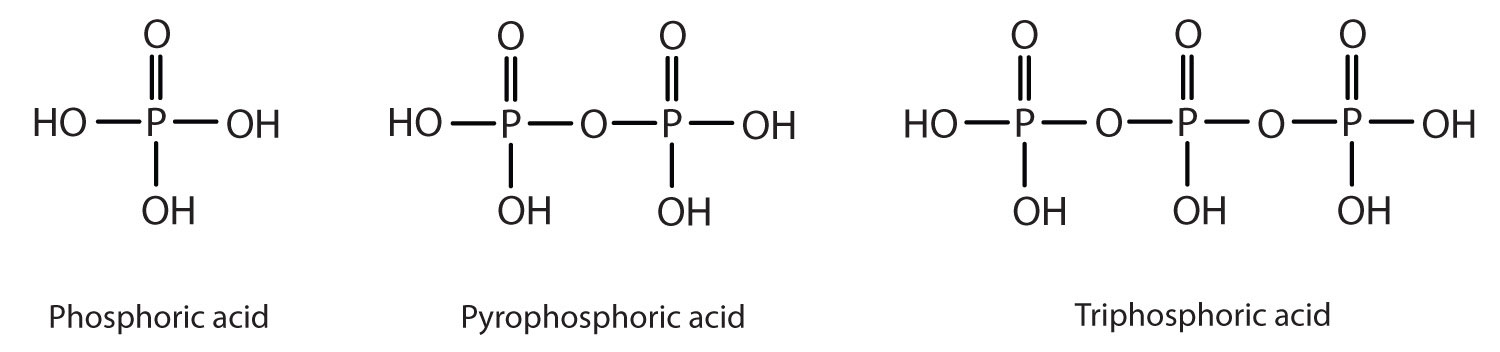
Esters of these acids are present in every plant and animal cell. They are biochemical intermediates in the transformation of food into usable energy. The bonds between phosphate units in adenosine triphosphate (ATP) are called phosphoanhydride bonds. These are high-energy bonds that store energy from the metabolism of foods. Hydrolysis of ATP releases energy as it is needed for biochemical processes (for instance, for muscle contraction). Phosphate esters are also important structural constituents of phospholipids and nucleic acids. (For more information about phospholipids and nucleic acids, see Chapter 7 "Lipids", Section 7.3 "Membranes and Membrane Lipids", and Chapter 10 "Nucleic Acids and Protein Synthesis", respectively.)
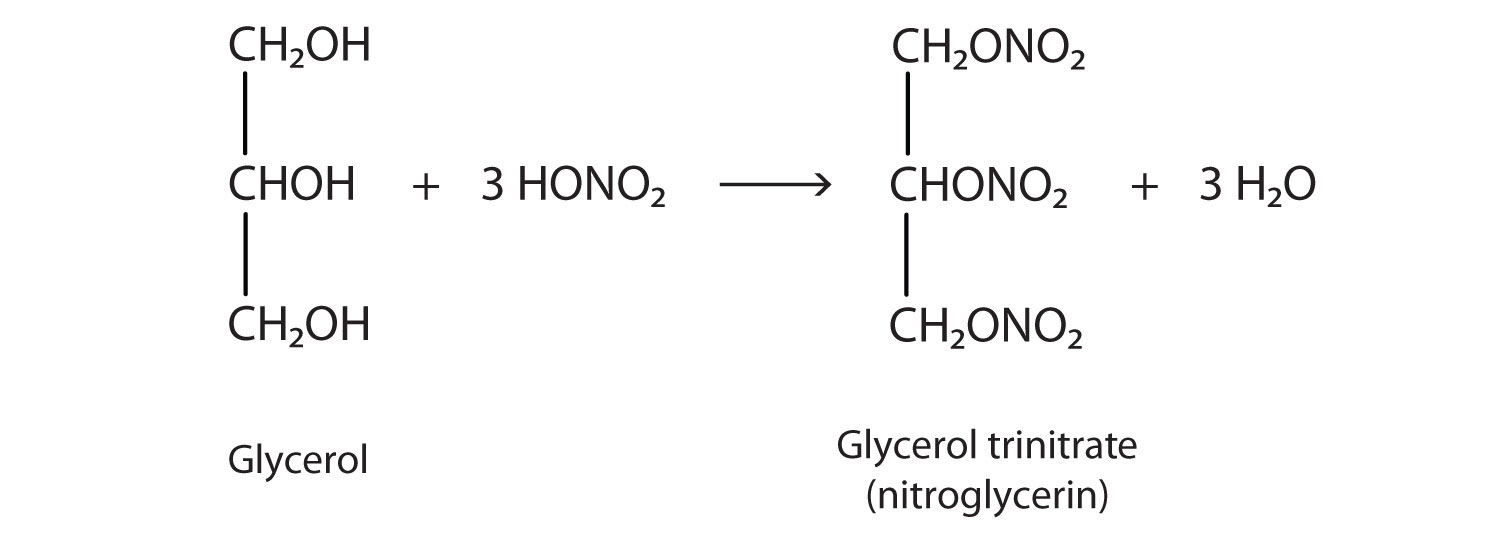
The explosive nitroglycerin (glyceryl trinitrate) is an ester formed from glycerol and nitric acid. It is used in medicine to relieve chest pain in heart disease.
What compounds combine to form phosphate esters?
phosphoric acids and alcohols
1. Draw the structure for each compound.
a. diethyl hydrogen phosphate
b. methyl dihydrogen phosphate
c. 1-glycerol phosphate
2. Name each compound.
a.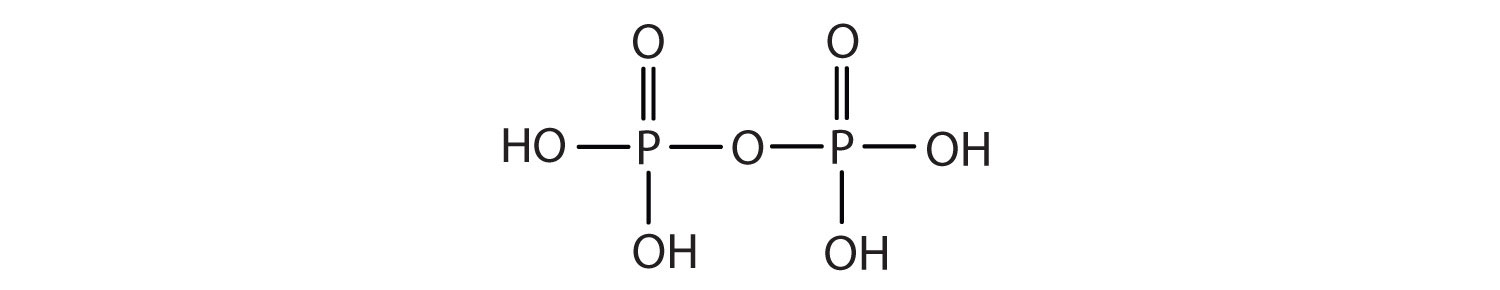
b.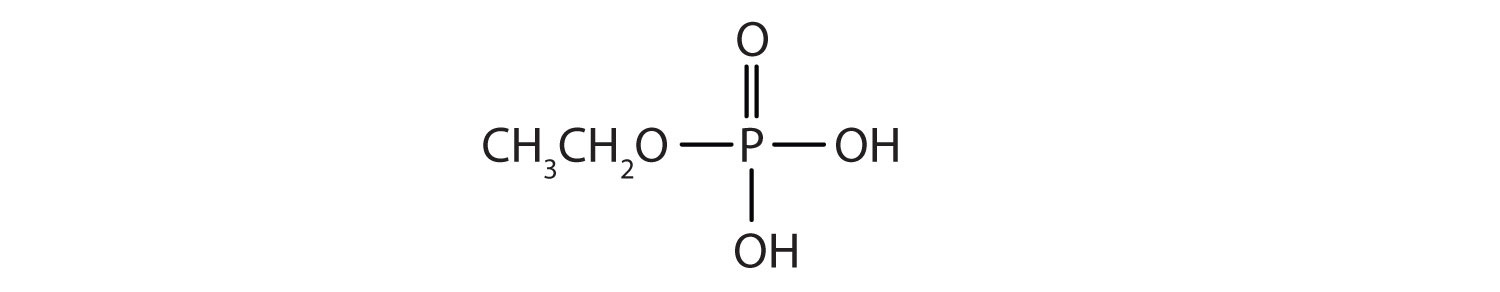
c.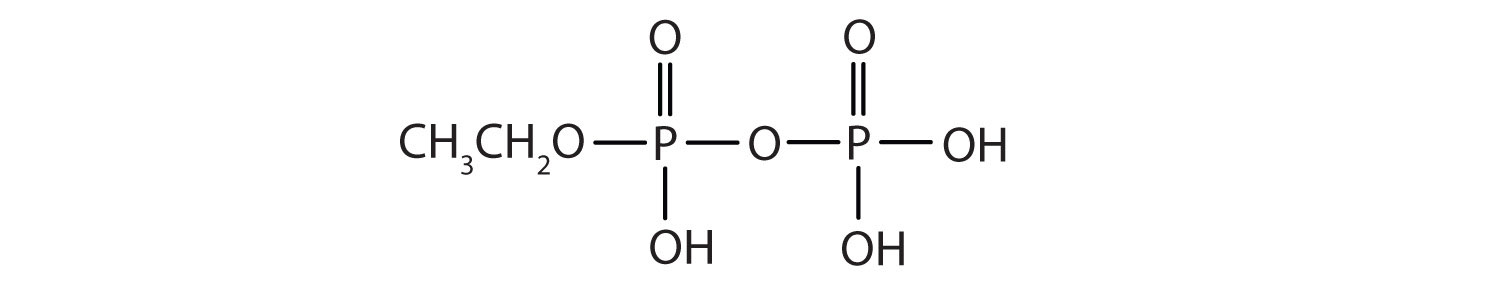
1.
a.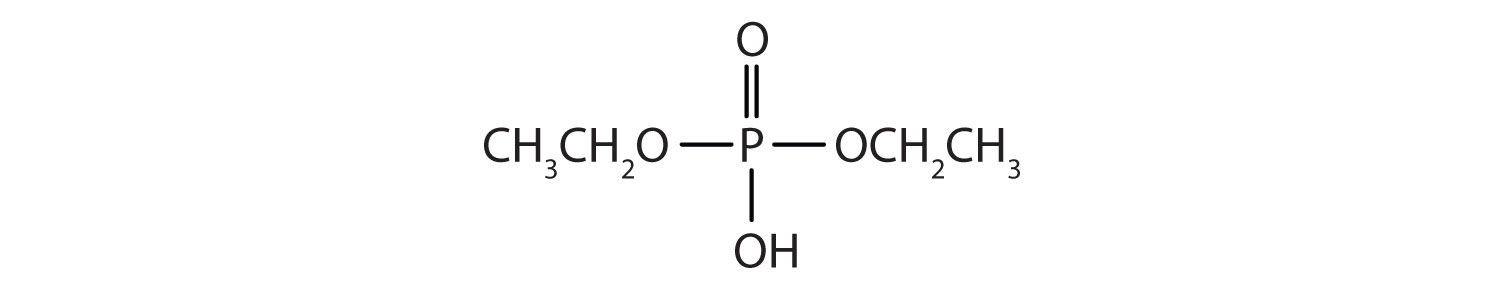
b.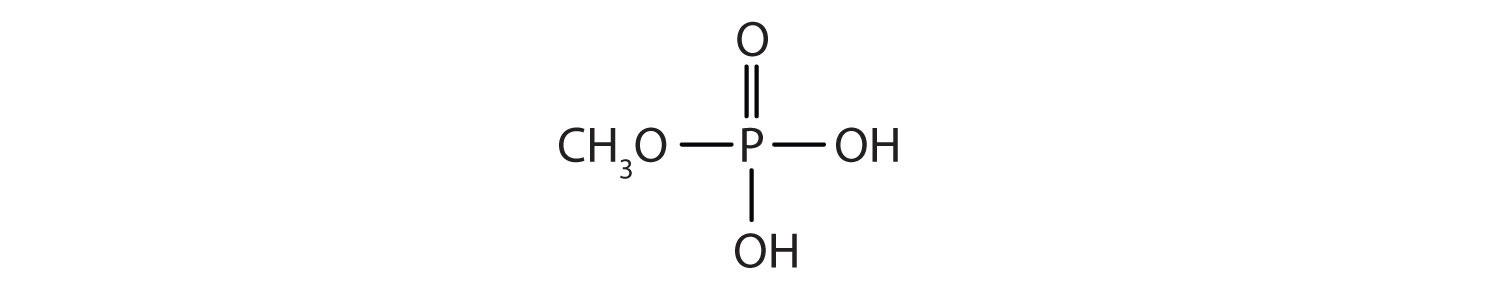
c.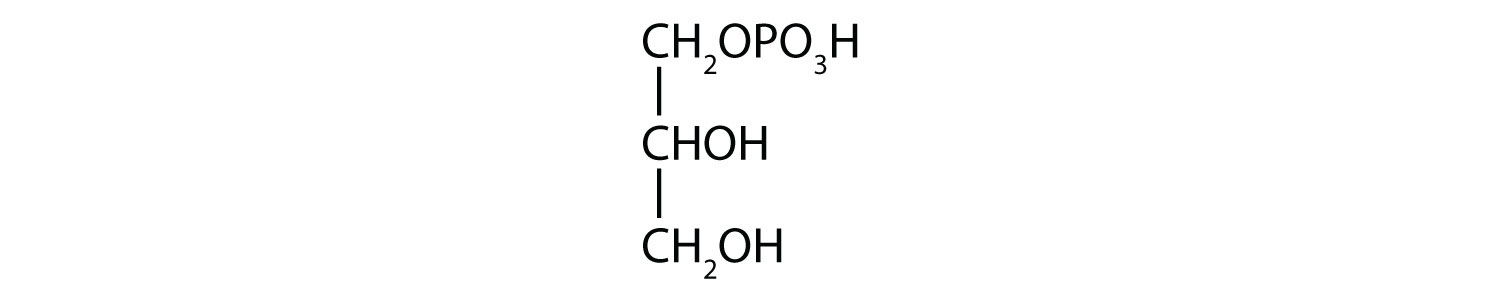
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
