Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball, John W. Hill, and Rhonda J. Scott
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
The next functional group we consider, the carbonyl group, has a carbon-to-oxygen double bond.
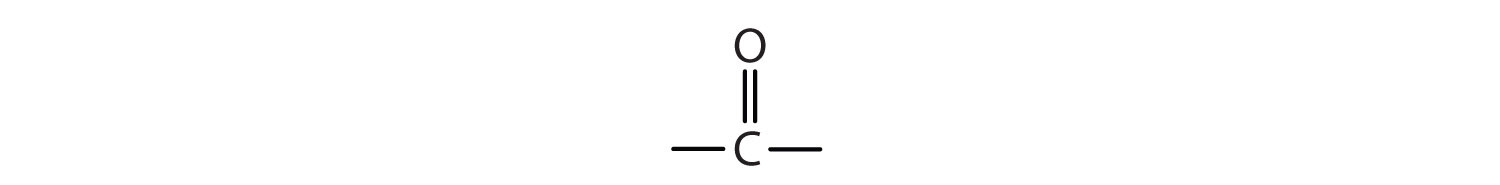
Carbonyl groups define two related families of organic compounds: the aldehydes and the ketones.
The carbonyl group is ubiquitous in biological compounds. It is found in carbohydrates, fats, proteins, nucleic acids, hormones, and vitamins—organic compounds critical to living systems.
In a ketone, two carbon groups are attached to the carbonyl carbon atom. The following general formulas, in which R represents an alkyl group and Ar stands for an aryl group, represent ketones.
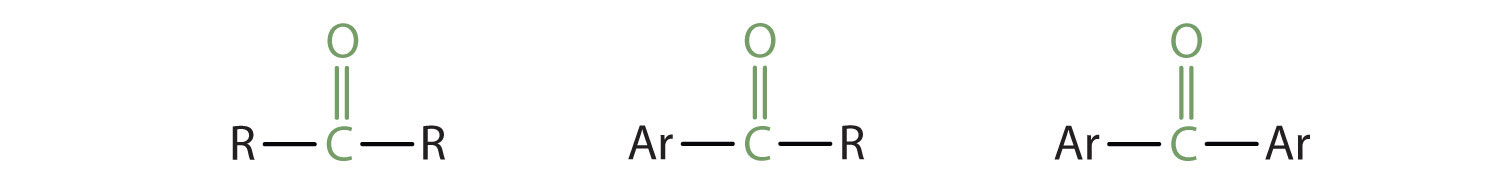
In an aldehyde, at least one of the attached groups must be a hydrogen atom. The following compounds are aldehydes:
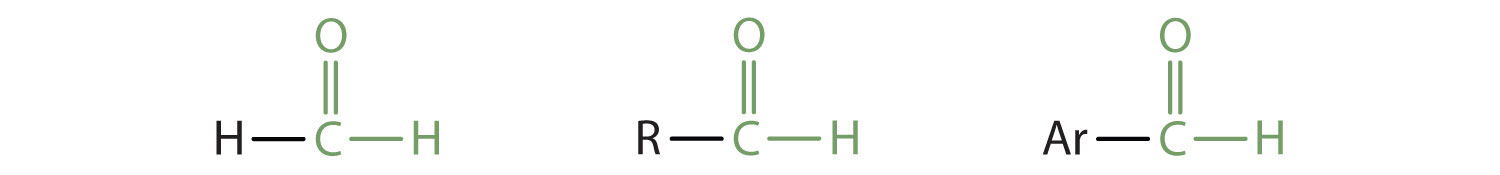
In condensed formulas, we use CHO to identify an aldehyde rather than COH, which might be confused with an alcohol. This follows the general rule that in condensed structural formulas H comes after the atom it is attached to (usually C, N, or O).

The carbon-to-oxygen double bond is not shown but understood to be present.
Because they contain the same functional group, aldehydes and ketones share many common properties, but they still differ enough to warrant their classification into two families.
Both common and International Union of Pure and Applied Chemistry (IUPAC) names are frequently used for aldehydes and ketones, with common names predominating for the lower homologs. The common names of aldehydes are taken from the names of the acids into which the aldehydes can be converted by oxidation. (For more information about carboxylic acids, see Chapter 4 "Carboxylic Acids, Esters", Section 4.2 "Carboxylic Acids: Structures and Names" through Section 4.4 "Physical Properties of Carboxylic Acids".)
The stems for the common names of the first four aldehydes are as follows:
Because the carbonyl group in a ketone must be attached to two carbon groups, the simplest ketone has three carbon atoms. It is widely known as acetone, a unique name unrelated to other common names for ketones.
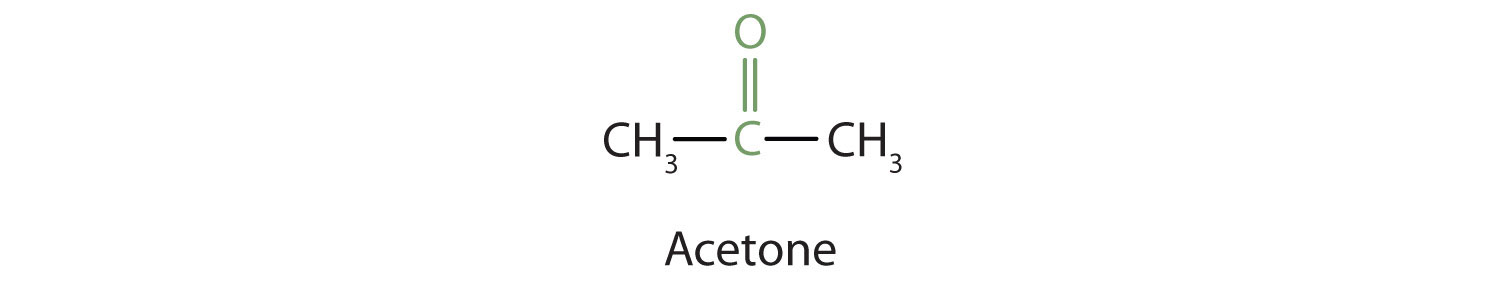
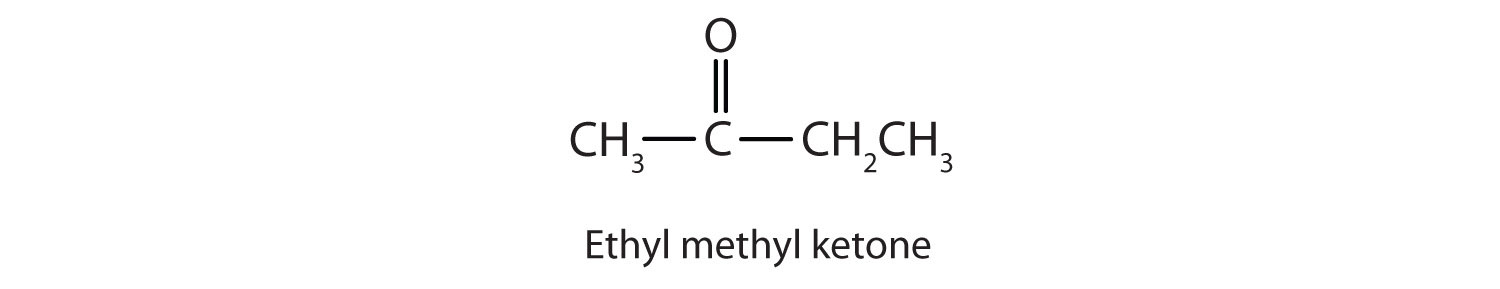
Generally, the common names of ketones consist of the names of the groups attached to the carbonyl group, followed by the word ketone. (Note the similarity to the naming of ethers.) Another name for acetone, then, is dimethyl ketone. The ketone with four carbon atoms is ethyl methyl ketone.
Classify each compound as an aldehyde or a ketone. Give the common name for each ketone.

Solution
Classify each compound as an aldehyde or a ketone. Give the common name for each ketone.
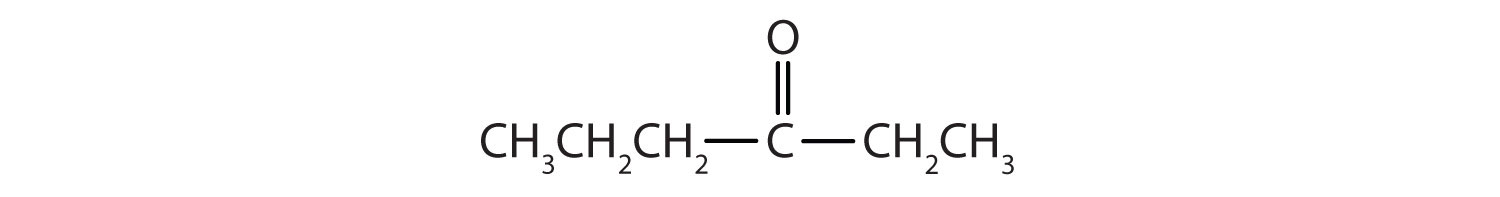
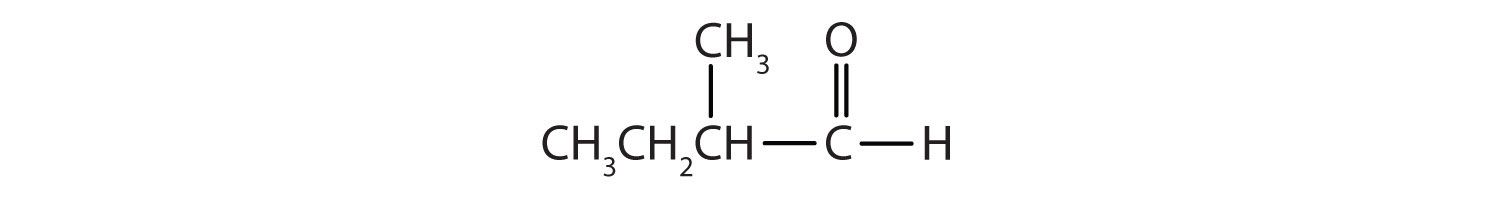
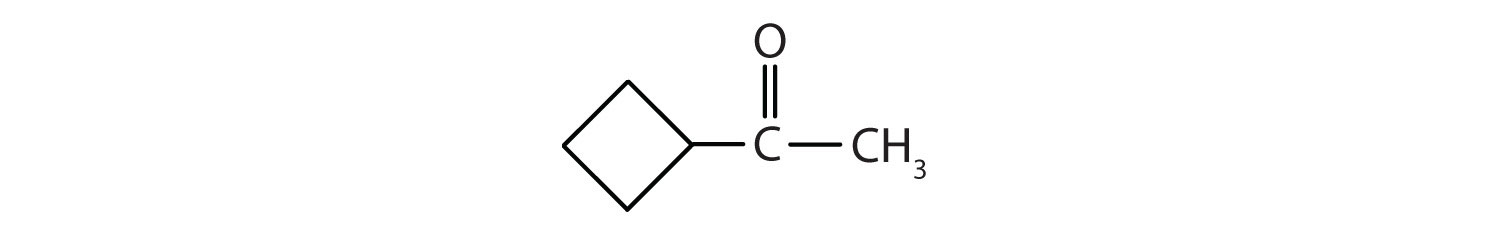
Here are some simple IUPAC rules for naming aldehydes and ketones:
Give the IUPAC name for each compound.
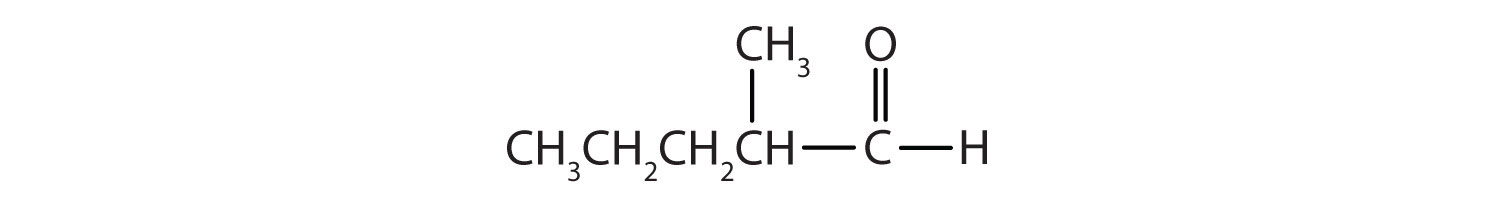
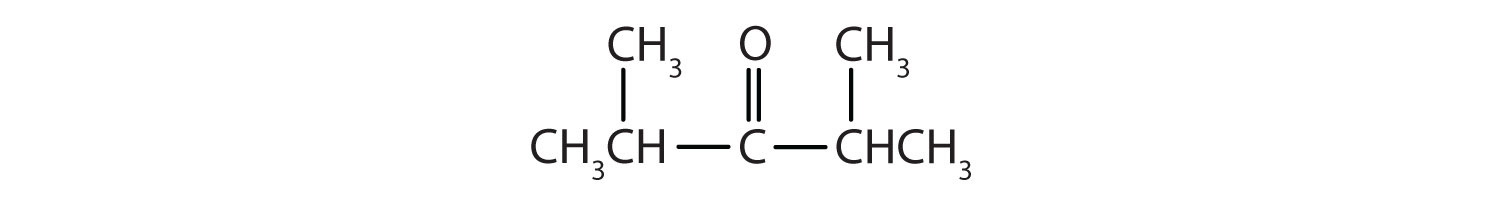
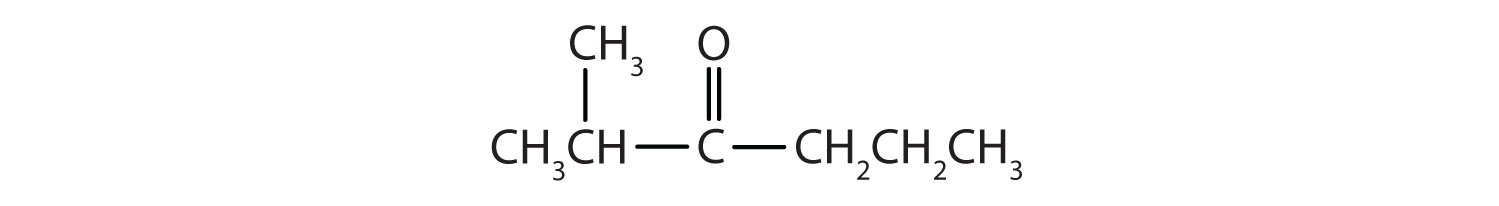
Solution
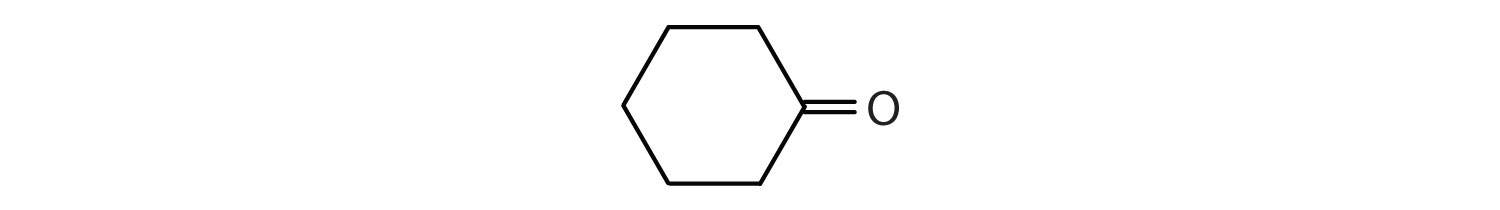
Give the IUPAC name for each compound.
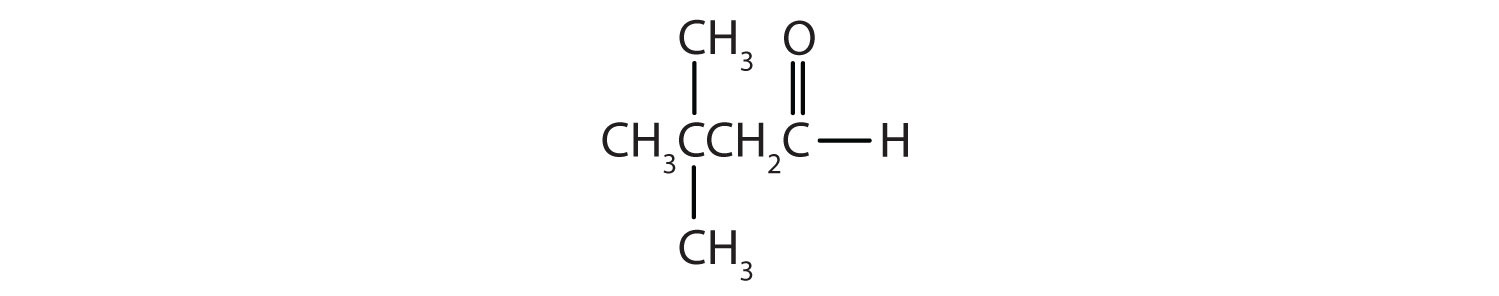
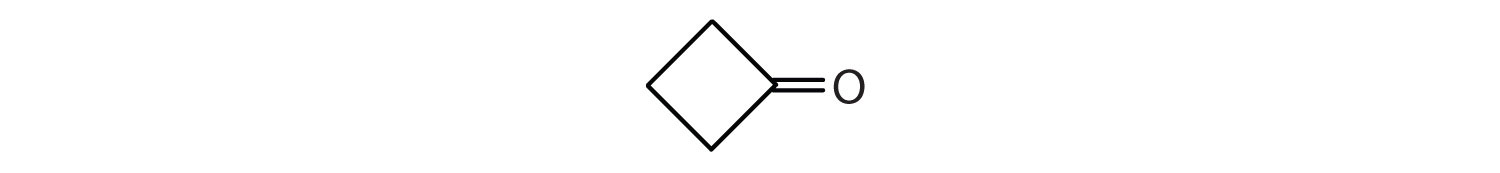
Draw the structure for each compound.
Solution
The octan- part of the name tells us that the LCC has eight carbon atoms. There is a chlorine (Cl) atom on the seventh carbon atom; numbering from the carbonyl group and counting the carbonyl carbon atom as C1, we place the Cl atom on the seventh carbon atom.
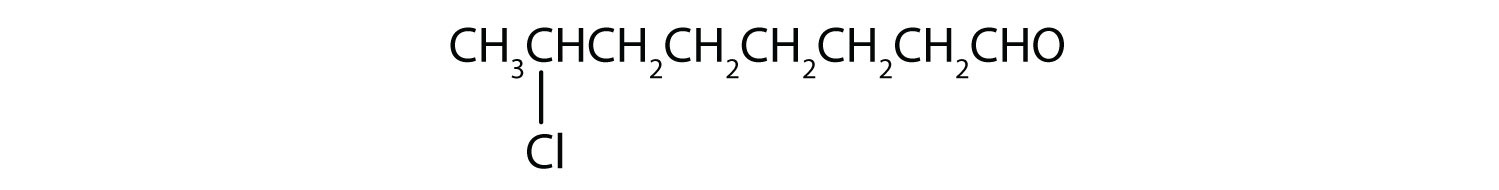
The hexan- part of the name tells us that the LCC has six carbon atoms. The 3 means that the carbonyl carbon atom is C3 in this chain, and the 4 tells us that there is a methyl (CH3) group at C4:
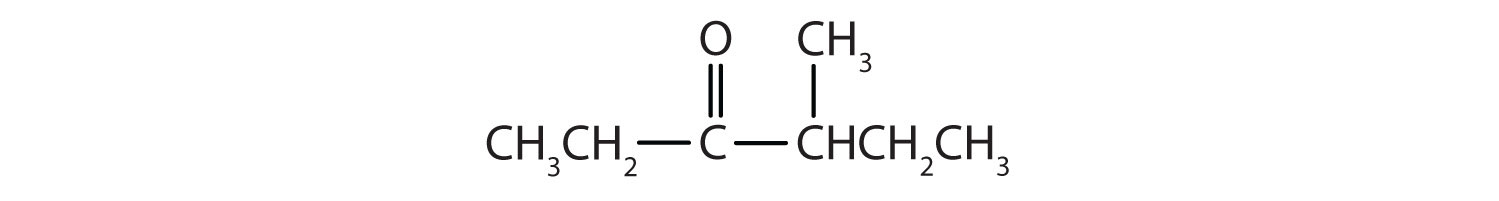
Draw the structure for each compound.
5-bromo-3-iodoheptanal
5-bromo-4-ethyl-2-heptanone
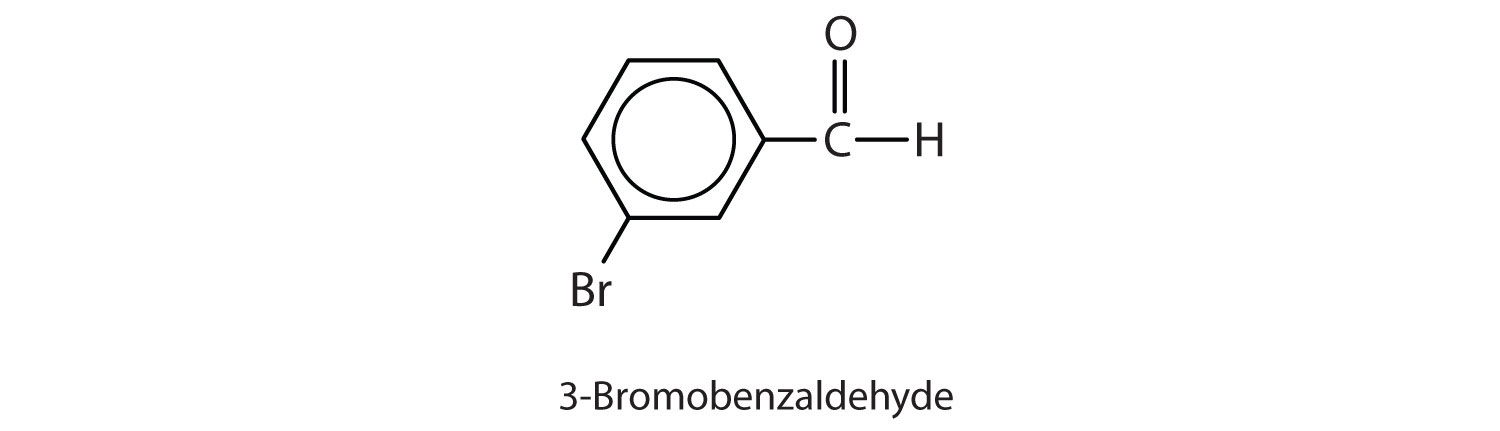
Give the structure and IUPAC name for the compound that has the common name m-bromobenzaldehyde (see Figure 3.2 "Some Interesting Aldehydes" for the structure of benzaldehyde).
Give the IUPAC name for glyceraldehyde, (HOCH2CHOHCHO). (Hint: as a substituent, the OH group is named hydroxy.)
2,3-dihydroxypropanal
1. Name each compound.
a.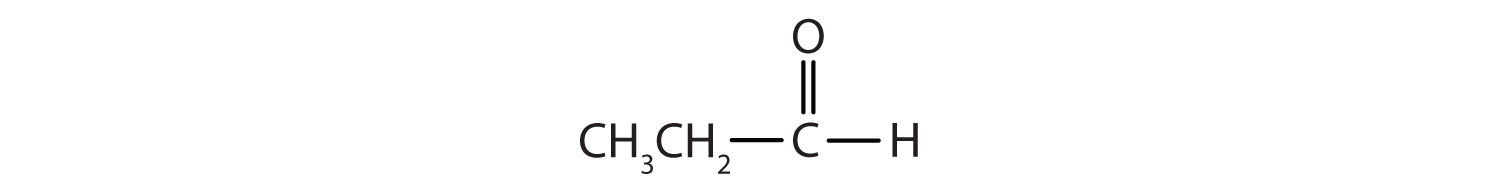
b.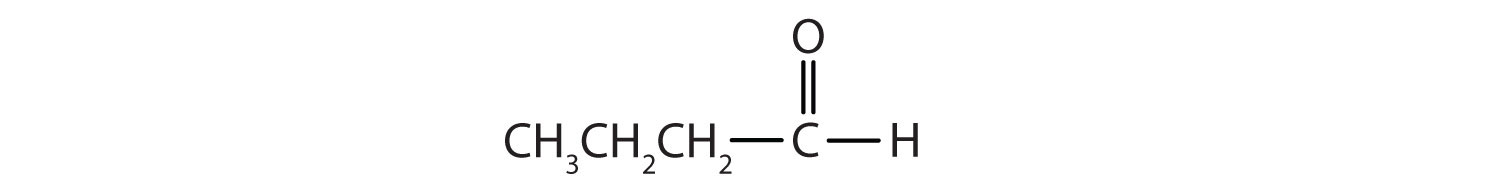
c.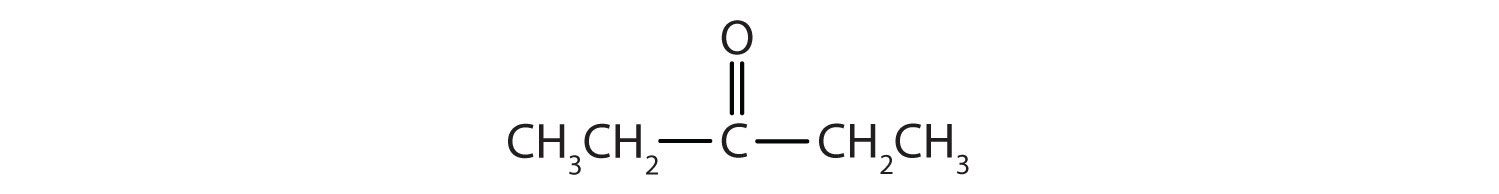
d.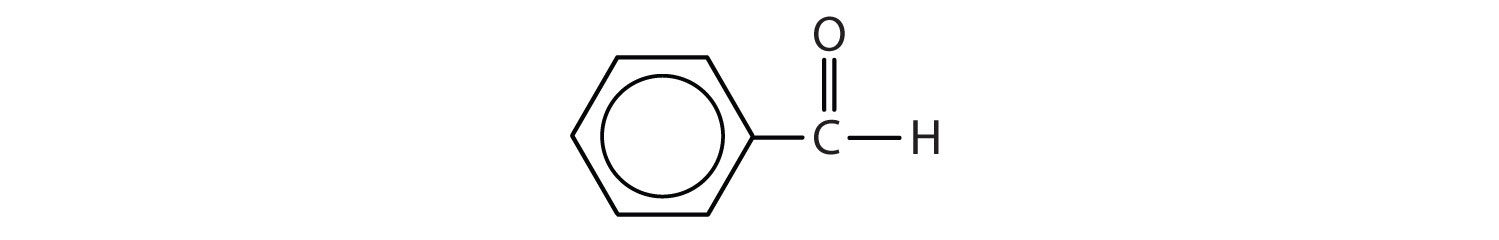
2. Name each compound.
a.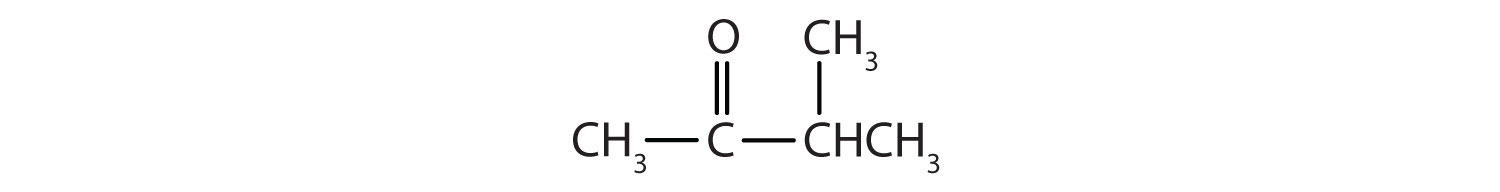
b. CH3CH2CH2CH2CH2CHO
c.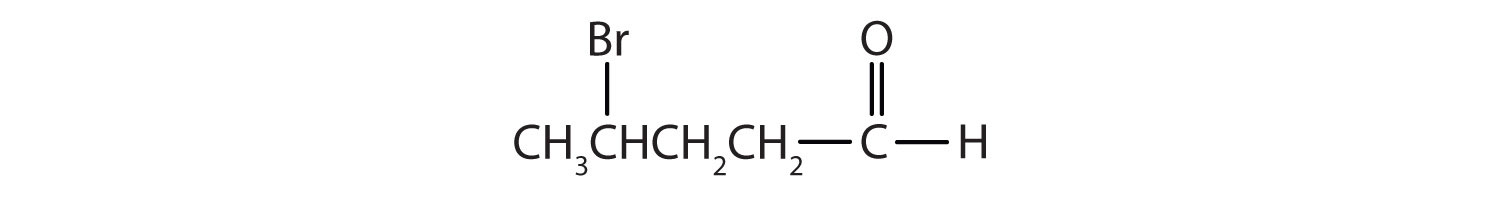
d.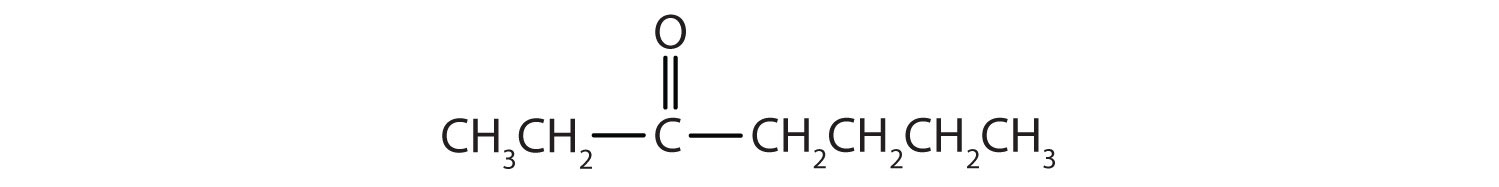
3. Draw the structure for each compound.
a. butyraldehyde
b. 2-hexanone
c. p-nitrobenzaldehyde
4. Draw the structure for each compound.
a. 5-ethyloctanal
b. 2-chloropropanal
c. 2-hydroxy-3-pentanone
1.
a. propanal or propionaldehyde
b. butanal or butyraldehyde
c. 3-pentanone or diethyl ketone
d. benzaldehyde
3.
a. CH3CH2CH2CHO
b.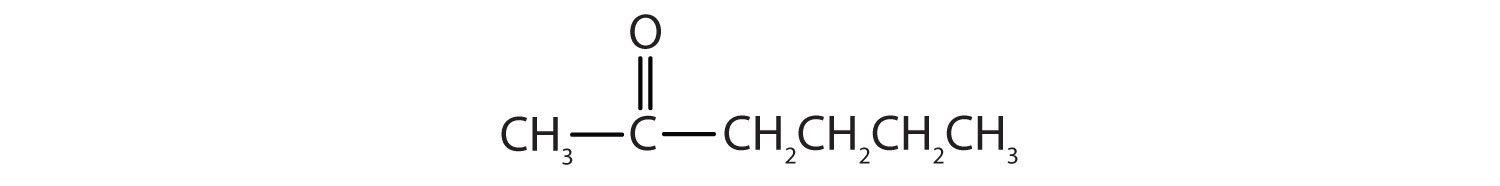
c.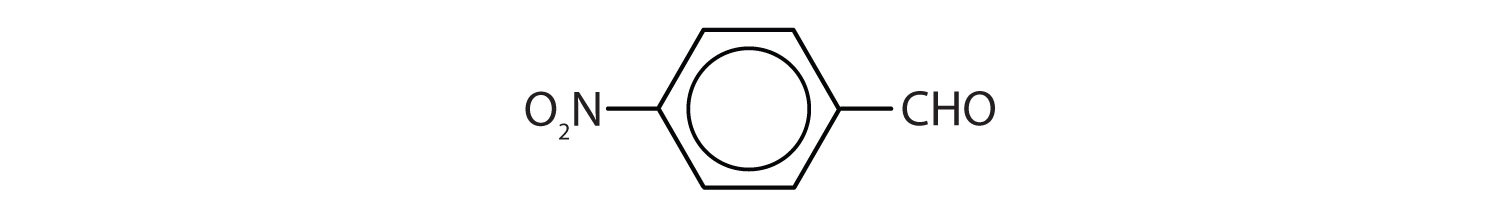
The carbon-to-oxygen double bond is quite polar, more polar than a carbon-to-oxygen single bond. The electronegative oxygen atom has a much greater attraction for the bonding electron pairs than does the carbon atom. The carbon atom has a partial positive charge, and the oxygen atom has a partial negative charge:
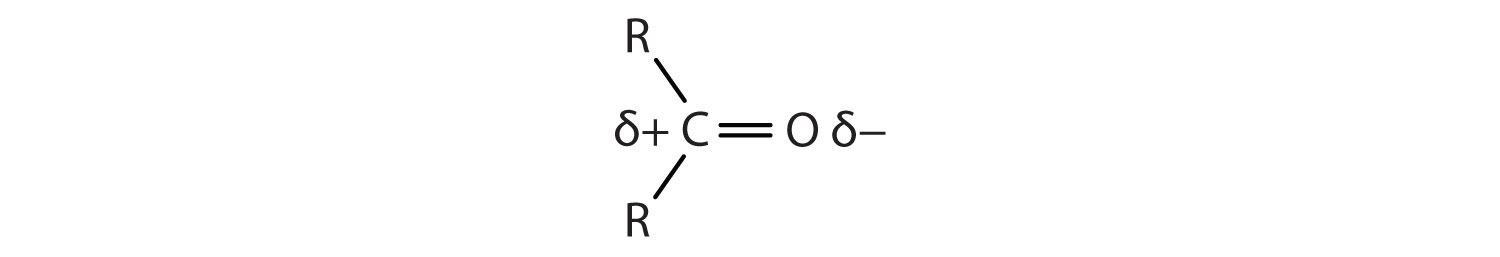
In aldehydes and ketones, this charge separation leads to dipole-dipole interactions that are great enough to significantly affect the boiling points. Table 3.1 "Boiling Points of Compounds Having Similar Molar Masses but Different Types of Intermolecular Forces" shows that the polar single bonds in ethers have little such effect, whereas hydrogen bonding between alcohol molecules is even stronger.
Table 3.1 Boiling Points of Compounds Having Similar Molar Masses but Different Types of Intermolecular Forces
| Compound | Family | Molar Mass | Type of Intermolecular Forces | Boiling Point (°C) |
|---|---|---|---|---|
| CH3CH2CH2CH3 | alkane | 58 | dispersion only | –1 |
| CH3OCH2CH3 | ether | 60 | weak dipole | 6 |
| CH3CH2CHO | aldehyde | 58 | strong dipole | 49 |
| CH3CH2CH2OH | alcohol | 60 | hydrogen bonding | 97 |
Formaldehyde is a gas at room temperature. Acetaldehyde boils at 20°C; in an open vessel, it boils away in a warm room. Most other common aldehydes are liquids at room temperature.
Although the lower members of the homologous series have pungent odors, many higher aldehydes have pleasant odors and are used in perfumes and artificial flavorings. As for the ketones, acetone has a pleasant odor, but most of the higher homologs have rather bland odors.
The oxygen atom of the carbonyl group engages in hydrogen bonding with a water molecule.
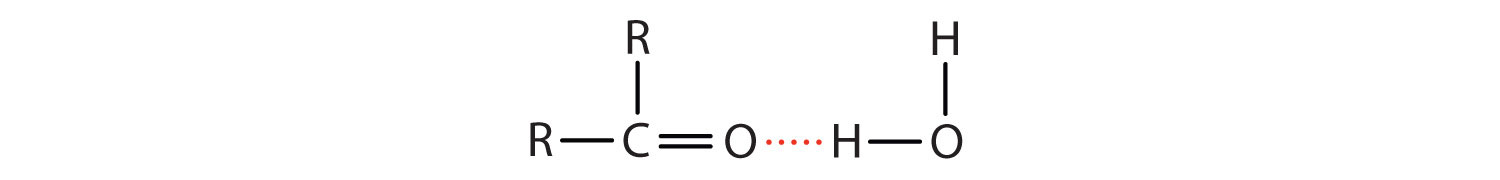
The solubility of aldehydes is therefore about the same as that of alcohols and ethers. Formaldehyde, acetaldehyde, and acetone are soluble in water. As the carbon chain increases in length, solubility in water decreases. The borderline of solubility occurs at about four carbon atoms per oxygen atom. All aldehydes and ketones are soluble in organic solvents and, in general, are less dense than water.
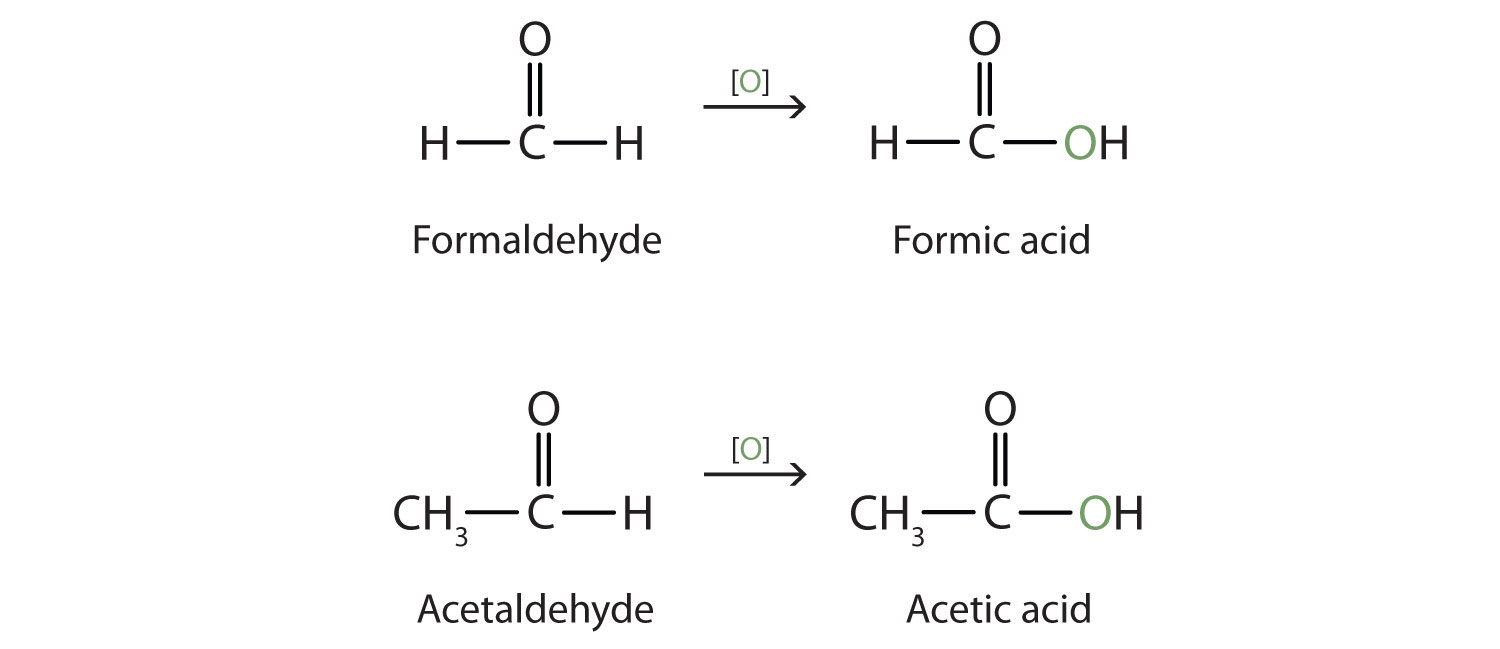
Aldehydes and ketones are much alike in many of their reactions, owing to the presence of the carbonyl functional group in both. They differ greatly, however, in one most important type of reaction: oxidation. Aldehydes are readily oxidized to carboxylic acids, whereas ketones resist oxidation.
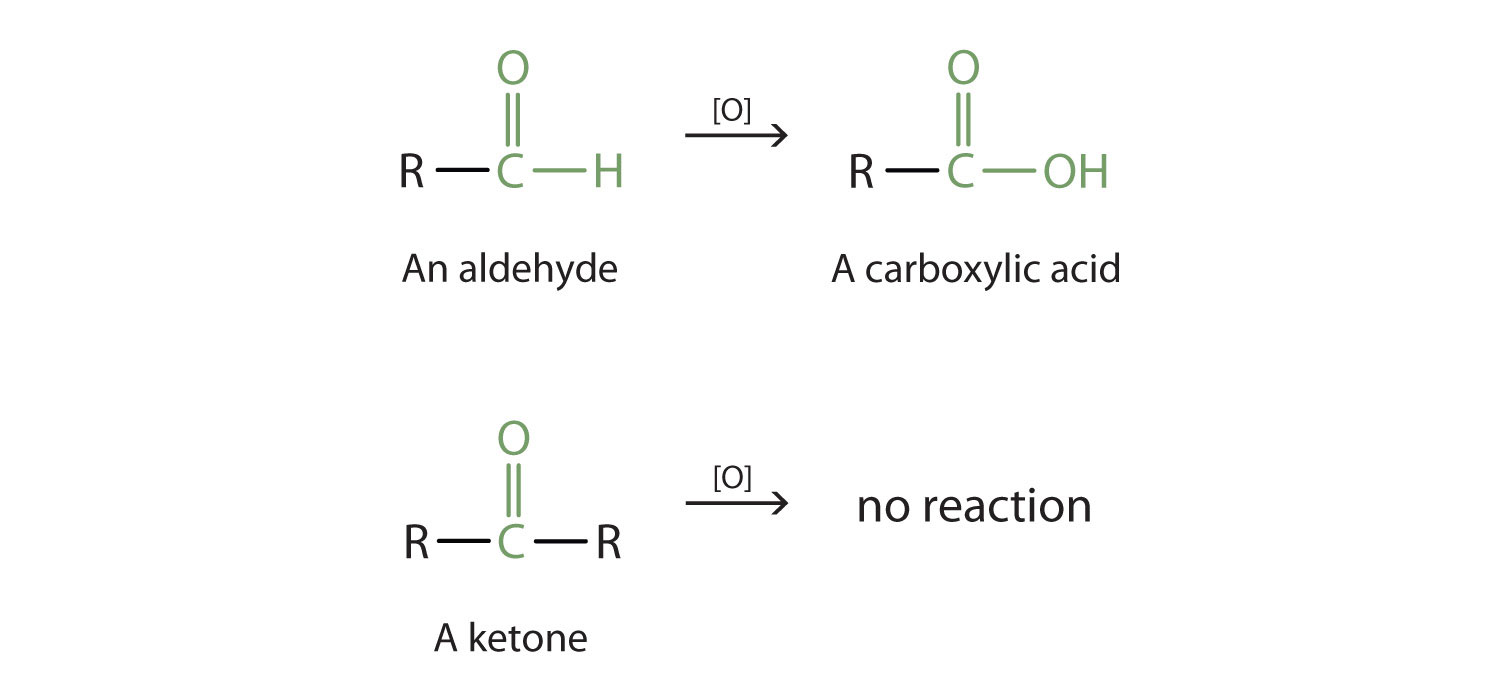
The aldehydes are, in fact, among the most easily oxidized of organic compounds. They are oxidized by oxygen (O2) in air to carboxylic acids.
2RCHO + O2 → 2RCOOHThe ease of oxidation helps chemists identify aldehydes. A sufficiently mild oxidizing agent can distinguish aldehydes not only from ketones but also from alcohols. Tollens’ reagent, for example, is an alkaline solution of silver (Ag+) ion complexed with ammonia (NH3), which keeps the Ag+ ion in solution.
H3N—Ag+—NH3When Tollens’ reagent oxidizes an aldehyde, the Ag+ ion is reduced to free silver (Ag).
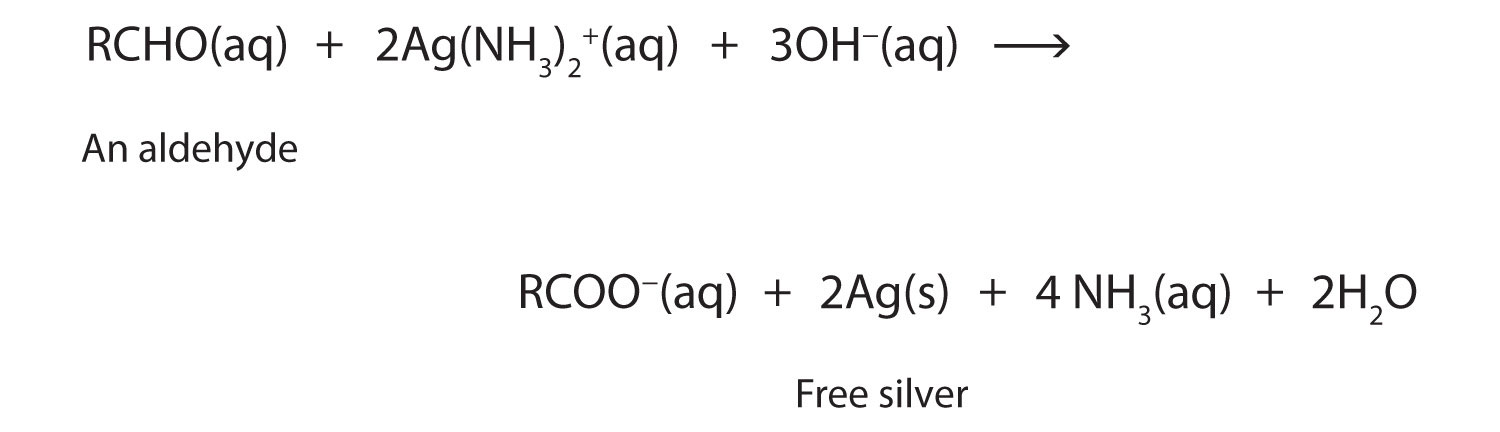
Deposited on a clean glass surface, the silver produces a mirror (Figure 3.1 "Aldehyde Reactions"). Ordinary ketones do not react with Tollens’ reagent.
Figure 3.1 Aldehyde Reactions

A reaction related to the Tollens’ reaction is often used to silver mirrors. These ornaments were silvered by such a reaction. Glucose, a simple sugar with an aldehyde functional group, is used as the reducing agent.
Source: Photo courtesy of Krebs Glas Lauscha, http://commons.wikimedia.org/wiki/File:Silvering.jpg.
Although ketones resist oxidation by ordinary laboratory oxidizing agents, they undergo combustion, as do aldehydes.
Formaldehyde has an irritating odor. Because of its reactivity, it is difficult to handle in the gaseous state. For many uses, it is therefore dissolved in water and sold as a 37% to 40% aqueous solution called formalin. Formaldehyde denatures proteins, rendering them insoluble in water and resistant to bacterial decay. (For more information about proteins, see Chapter 9 "Proteins, and Enzymes", Section 9.1 "Proteins".) For this reason, formalin is used in embalming solutions and in preserving biological specimens.
Aldehydes are the active components in many other familiar substances. Large quantities of formaldehyde are used to make phenol-formaldehyde resins for gluing the wood sheets in plywood and as adhesives in other building materials. Sometimes the formaldehyde escapes from the materials and causes health problems in some people. While some people seem unaffected, others experience coughing, wheezing, eye irritation, and other symptoms.
Acetaldehyde is an extremely volatile, colorless liquid. It is a starting material for the preparation of many other organic compounds. Acetaldehyde is formed as a metabolite in the fermentation of sugars and in the detoxification of alcohol in the liver. Aldehydes are the active components of many other familiar materials (Figure 3.2 "Some Interesting Aldehydes").
The odor of green leaves is due in part to a carbonyl compound, cis-3-hexenal, which with related compounds is used to impart a “green” herbal odor to shampoos and other products.
Figure 3.2 Some Interesting Aldehydes
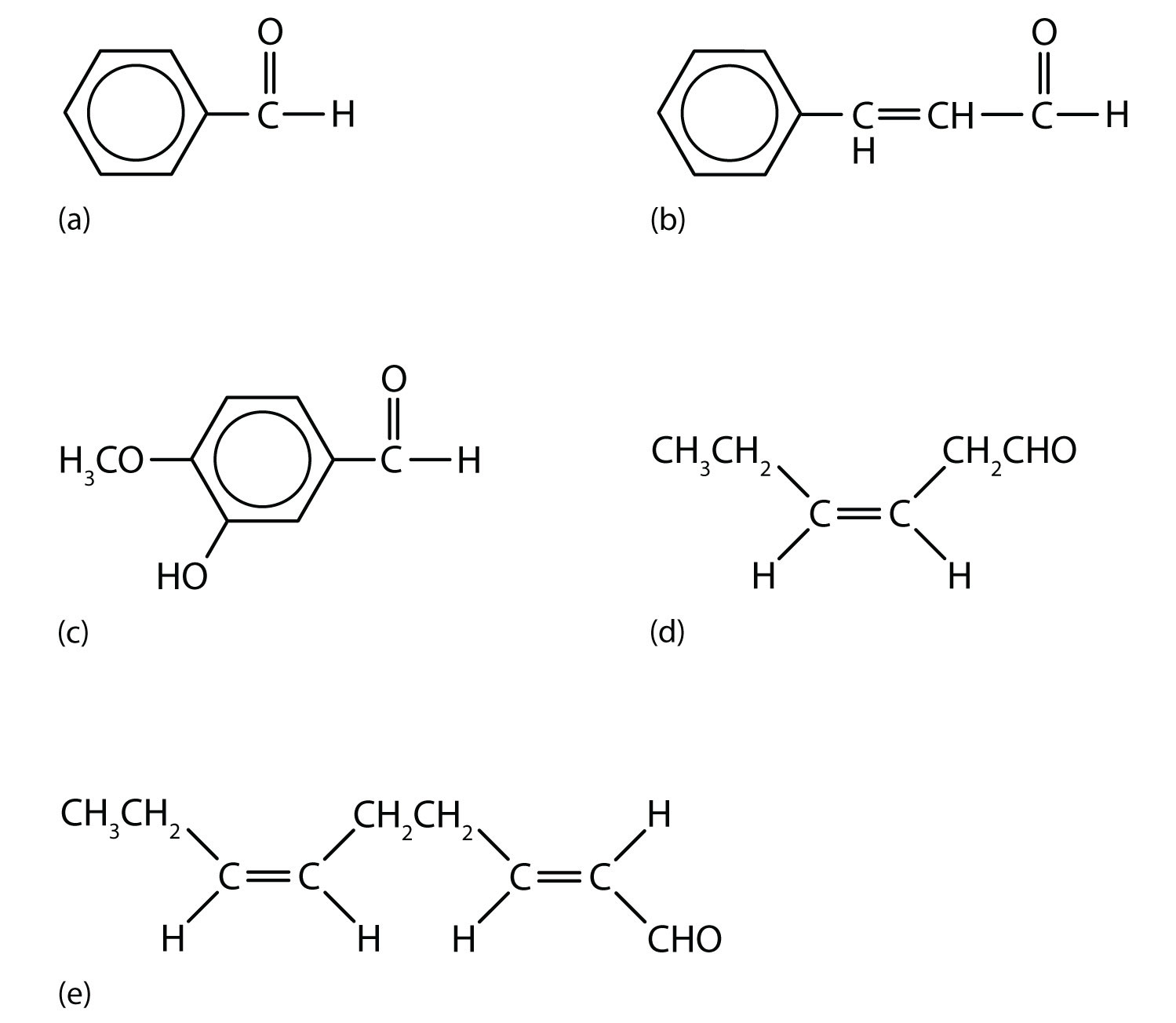
(a) Benzaldehyde is an oil found in almonds; (b) cinnamaldehyde is oil of cinnamon; (c) vanillin gives vanilla its flavor; (d) cis-3-hexenal provides an herbal odor; and (e) trans-2-cis-6-nonadienal gives a cucumber odor.
Acetone is the simplest and most important ketone. Because it is miscible with water as well as with most organic solvents, its chief use is as an industrial solvent (for example, for paints and lacquers). It is also the chief ingredient in some brands of nail polish remover.
Acetone is formed in the human body as a by-product of lipid metabolism. (For more information about metabolic reactions, see Chapter 11 "Metabolic Pathways and Energy Production".) Normally, acetone does not accumulate to an appreciable extent because it is oxidized to carbon dioxide and water. The normal concentration of acetone in the human body is less than 1 mg/100 mL of blood. In certain disease states, such as uncontrolled diabetes mellitus, the acetone concentration rises to higher levels. It is then excreted in the urine, where it is easily detected. In severe cases, its odor can be noted on the breath.
Ketones are also the active components of other familiar substances, some of which are noted in the accompanying figure.
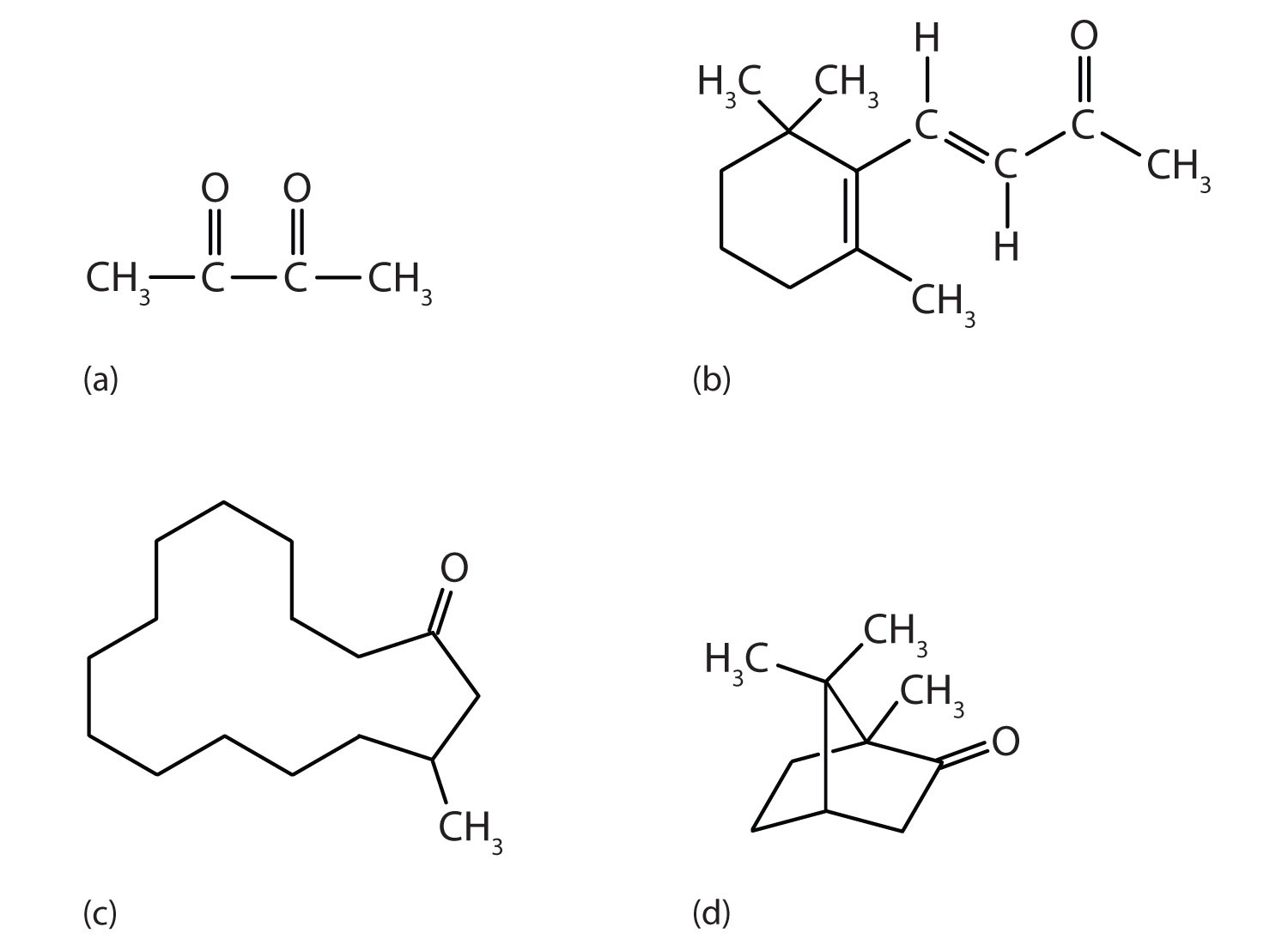
Some ketones have interesting properties: (a) Butter flavoring comes from 2,3-butanedione; (b) β-ionone is responsible for the odor of violets; (c) muscone is musk oil, an ingredient in perfumes; and (d) camphor is used in some insect repellents.
Certain steroid hormones have the ketone functional group as a part of their structure. Two examples are progesterone, a hormone secreted by the ovaries that stimulates the growth of cells in the uterine wall and prepares it for attachment of a fertilized egg, and testosterone, the main male sex hormone. These and other sex hormones affect our development and our lives in fundamental ways. (For more information about the sex hormones, see Chapter 7 "Lipids", Section 7.4 "Steroids".)
What feature of their structure makes aldehydes easier to oxidize than ketones?
How does the carbon-to-oxygen bond of aldehydes and ketones differ from the carbon-to-carbon bond of alkenes?
the H on the carbonyl carbon atom
The carbon-to-oxygen double bond is polar; the carbon-to-carbon double bond is nonpolar.
1. Which compound in each pair has the higher boiling point?
a. acetone or 2-propanol
b. dimethyl ether or acetaldehyde
2. Which compound in each pair has the higher boiling point?
a. butanal or 1-butanol
b. acetone or isobutane
3. Draw the structure of the alcohol that could be oxidized to each compound.
a. cyclohexanone
b. 2-methyl-1-propanal
4. Draw the structure of the alcohol that could be oxidized to each compound.
a. 2-pentanone
b. o-methylbenzaldehyde
5. Acetaldehyde is treated with each substance.
a. Ag+(aq)—What inorganic product, if any, is formed?
b. K2Cr2O7 in an acid solution—What organic product, if any, is formed?
6. Acetone is treated with each substance.
a. Ag+(aq) —What inorganic product, if any, is formed?
b. K2Cr2O7 in an acid solution—What organic product, if any, is formed?
1.
a. 2-propanol
b. acetaldehyde
3.
a.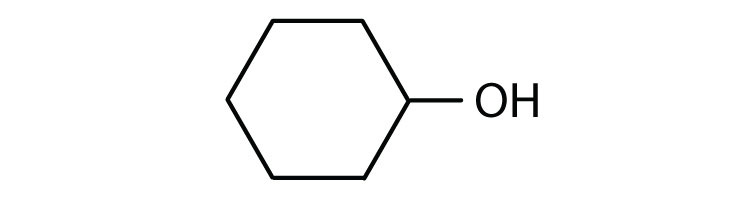
b.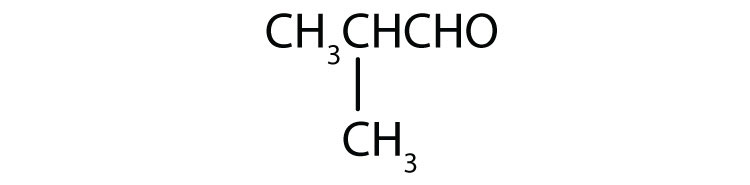
5.
a. silver metal (Ag)
b. acetic acid (CH3COOH)
Because sulfur is in the same group (6A) of the periodic table as oxygen, the two elements have some similar properties. We might expect sulfur to form organic compounds related to those of oxygen, and indeed it does.
Thiols (also called mercaptans), which are sulfur analogs of alcohols, have the general formula RSH. Methanethiol (also called methyl mercaptan), has the formula CH3SH. Ethanethiol (ethyl mercaptan) is the most common odorant for liquid propane (LP) gas.
The mild oxidation of thiols gives compounds called disulfides.
As we note in Chapter 9 "Proteins, and Enzymes", Section 9.1 "Proteins", the amino acids cysteine [HSCH2CH(NH2)COOH] and methionine [CH3SCH2CH2CH(NH2)COOH] contain sulfur atoms, as do all proteins that contain these amino acids. Disulfide linkages (–S–S–) between protein chains are extremely important in protein structure.
Thioethers, which are sulfur analogs of ethers, have the form general formula RSR′. An example is dimethylsulfide (CH3SCH3), which is responsible for the sometimes unpleasant odor of cooking cabbage and related vegetables. Note that methionine has a thioether functional group.
Paramedics are highly trained experts at providing emergency medical treatment. Their critical duties often include rescue work and emergency medical procedures in a wide variety of settings, sometimes under extremely harsh and difficult conditions. Like other science-based professions, their work requires knowledge, ingenuity, and complex thinking, as well as a great deal of technical skill. The recommended courses for preparation in this field include anatomy, physiology, medical terminology, and—not surprisingly—chemistry. An understanding of basic principles of organic chemistry, for example, is useful when paramedics have to deal with such traumas as burns from fuel (hydrocarbons) or solvent (alcohols, ethers, esters, and so on) fires and alcohol and drug overdoses.
To become a paramedic requires 2–4 y of training and usually includes a stint as an emergency medical technician (EMT). An EMT provides basic care, can administer certain medications and treatments, such as oxygen for respiratory problems and epinephrine (adrenalin) for allergic reactions, and has some knowledge of common medical conditions. A paramedic, in contrast, must have extensive knowledge of common medical problems and be trained to administer a wide variety of emergency drugs.
Paramedics usually work under the direction of a medical doctor with a title such as “medical director.” Some paramedics are employed by fire departments and may work from a fire engine that carries medical equipment as well as fire-fighting gear. Some work from hospital-sponsored ambulances and continue to care for their patients after reaching the hospital emergency room. Still other paramedics work for a government department responsible for emergency health care in a specific geographical area. Finally, some work for private companies that contract to provide service for a government body.
An experienced paramedic has a broad range of employment options, including training for mountain or ocean rescue, working with police department special weapons and tactics (SWAT) teams, or working in isolated settings such as on oil rigs. With their expertise at treating and stabilizing patients before quickly moving them to a hospital, paramedics often provide the first critical steps in saving an endangered life. The following quotation, inscribed on the Arlington National Cemetery headstone of Army Lieutenant R. Adams Cowley, who is often called the “father” of shock trauma medicine, serves as the motto for many paramedic units: “Next to creating a life the finest thing a man can do is save one.” —Abraham Lincoln
What is the functional group of a thiol? Write the condensed structural formula for ethanethiol (ethyl mercaptan).
What is the functional group of a disulfide? Write the condensed structural formula for dipropyl disulfide.
SH; CH3CH2SH
–S–S–; CH3CH2CH2SSCH2CH2CH3
A common natural gas odorant is tert-butyl mercaptan. What is its condensed structural formula?
Write the equation for the oxidation of ethanethiol to diethyl disulfide.
(CH3)3CSH
To ensure that you understand the material in this chapter, you should review the meanings of the following bold terms in the summary and ask yourself how they relate to the topics in the chapter.
A functional group is any atom or atom group that confers characteristic properties to a family of compounds.
The hydroxyl group (OH) is the functional group of the alcohols. The alcohols are represented by the general formula ROH. Alcohols are derived from alkanes by replacing one or more hydrogen atoms by an OH group. A primary (1°) alcohol (RCH2OH) has the OH group on a carbon atom attached to one other carbon atom; a secondary (2°) alcohol (R2CHOH) has the OH group on a carbon atom attached to two other carbon atoms; and a tertiary (3°) alcohol (R3COH) has the OH group on a carbon atom attached to three other carbon atoms.
The ability to engage in hydrogen bonding greatly increases the boiling points of alcohols compared to hydrocarbons of comparable molar mass. Alcohols can also engage in hydrogen bonding with water molecules, and those with up to about four carbon atoms are soluble in water.
Many alcohols can be synthesized by the hydration of alkenes. Common alcohols include methanol, ethanol, and isopropyl alcohol. Methanol is quite poisonous. It can cause blindness or even death. Ethanol can be prepared from ethylene or made by fermentation. It is the “alcohol” in alcoholic beverages. On occasion, people drink methanol by mistake, thinking it is the beverage alcohol. On occasion, unscrupulous bootleggers, sell methanol to unsuspecting customers. In either case, the results are often tragic.
Rubbing alcohol is usually a 70% aqueous solution of isopropyl alcohol. Ethanol is also used in some rubbing alcohol formulations.
When water is removed from an alcohol in a dehydration step, the result is either an alkene or an ether, depending on the reaction conditions. Primary alcohols are oxidized to aldehydes or carboxylic acids, and secondary alcohols are oxidized to ketones. Tertiary alcohols are not easily oxidized.
Alcohols containing two OH groups on adjacent carbon atoms are called glycols.
Phenols (ArOH) are compounds having the OH group attached to an aromatic ring.
Ethers (ROR′, ROAr, ArOAr) are compounds in which an oxygen atom is joined to two organic groups.
The carbonyl group, a carbon-to-oxygen double bond, is the defining feature of aldehydes and ketones. In aldehydes at least one bond on the carbonyl group is a carbon-to-hydrogen bond; in ketones, both available bonds on the carbonyl carbon atom are carbon-to-carbon bonds. Aldehydes are synthesized by the oxidation of primary alcohols. The aldehyde can be further oxidized to a carboxylic acid. Ketones are prepared by the oxidation of secondary alcohols. Mild oxidizing agents oxidize aldehydes to carboxylic acids. Ketones are not oxidized by these reagents.
A thiol is a compound with an SH functional group.
1. Describe two ways that ethanol can be prepared. Which method is used to produce alcoholic beverages?
2. Give the structure of the alkene from which isopropyl alcohol is made by reaction with water in an acidic solution.
3. Ethanol is used as a solvent for some drugs that are not soluble in water. Why is methanol not used in medicines?
4. Give the structure of the alkene that is made from tert-butyl alcohol [(CH3)3COH] by reaction with water in an acidic solution.
5. Classify each conversion as oxidation, dehydration, or hydration (only the organic starting material and product are shown):
a. CH3OH → HCHO
b. CH3CHOHCH3 → CH3CH=CH2
c. CH2=CHCH2CH3 → CH3CHOHCH2CH3
6. Classify each conversion as oxidation, dehydration, or hydration (only the organic starting material and product are shown.):
a. CH3CHOHCH3 → CH3COCH3
b. HOOCCH=CHCOOH → HOOCCH2CHOHCOOH
c. 2 CH3OH → CH3OCH3
7. Why is methanol so much more toxic to humans than ethanol?
8. Each of the four isomeric butyl alcohols is treated with potassium dichromate (K2Cr2O7) in acid. Give the product (if any) expected from each reaction.
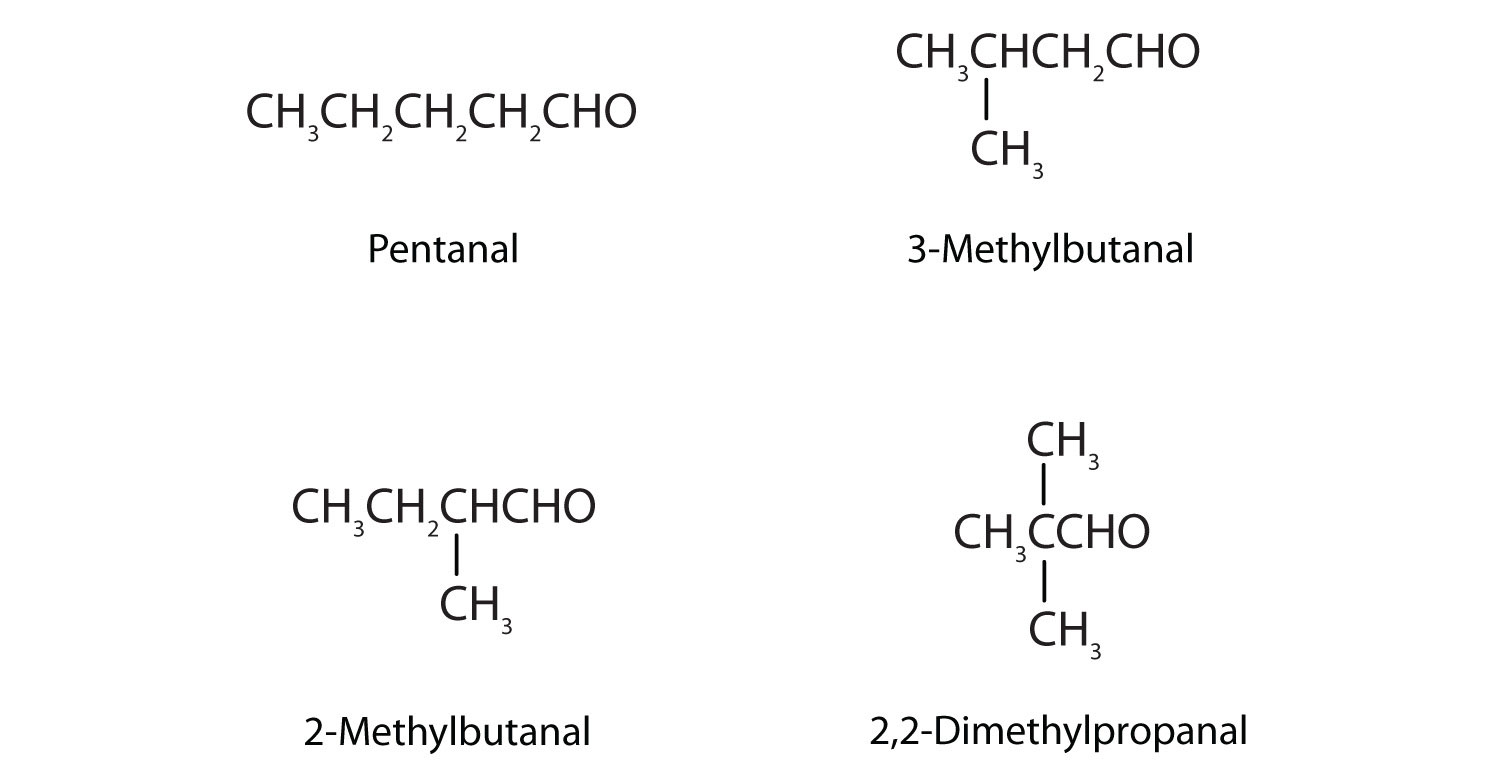
9. Draw the structures and give IUPAC names for the four isomeric aldehydes having the formula C5H10O.
10. Write an equation for the reaction of phenol with aqueous NaOH.
11. Write an equation for the ionization of phenol in water.
12. Draw the structures and give the common and IUPAC names for the three isomeric ketones having the formula C5H10O.
13. As we shall see in Chapter 6 "Carbohydrates", 2,3-dihydroxypropanal and 1,3-dihydroxyacetone are important carbohydrates. Draw their structures.
14. Glutaraldehyde (pentanedial) is a germicide that is replacing formaldehyde as a sterilizing agent. It is less irritating to the eyes, the nose, and skin. Write the condensed structural formula of glutaraldehyde.
15. Why does the oxidation of isopropyl alcohol give a ketone, whereas the oxidation of isobutyl alcohol gives an aldehyde?
16. Identify each compound as an alcohol, a phenol, or an ether. Classify any alcohols as primary (1°), secondary (2°), or tertiary (3°).
a. CH3CH2CH2OH
b.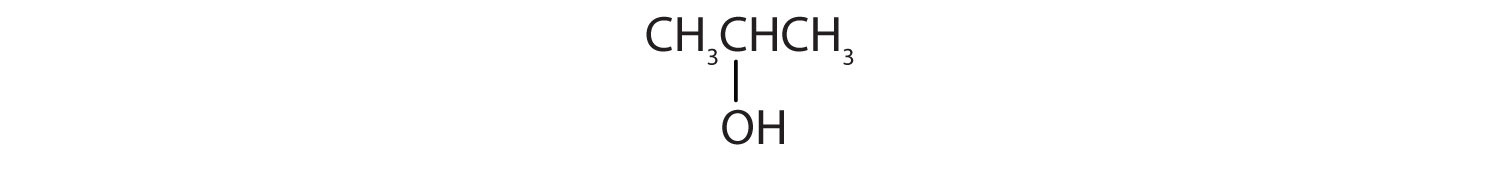
c.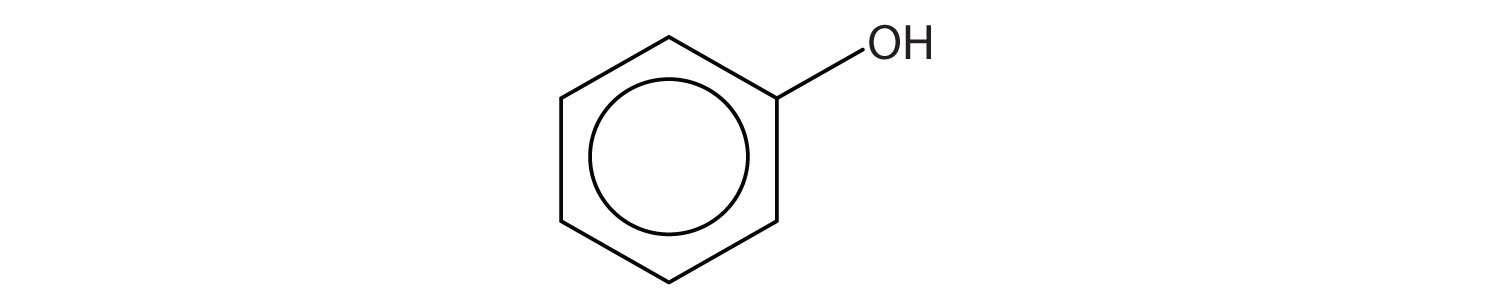
d.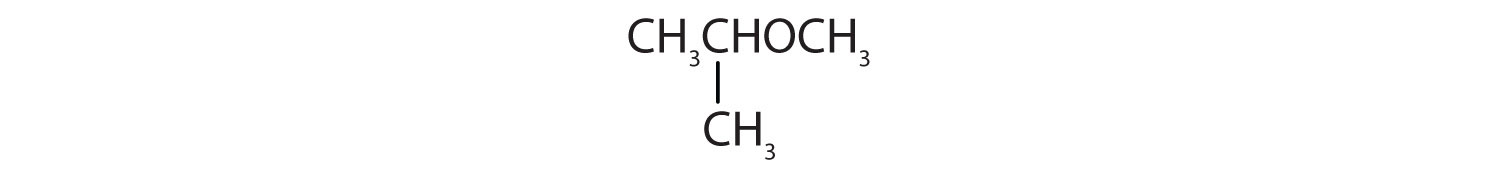
17. Identify each compound as an alcohol, a phenol, or an ether. Classify any alcohols as primary, secondary, or tertiary.
a. CH3CH2OCH2CH3
b.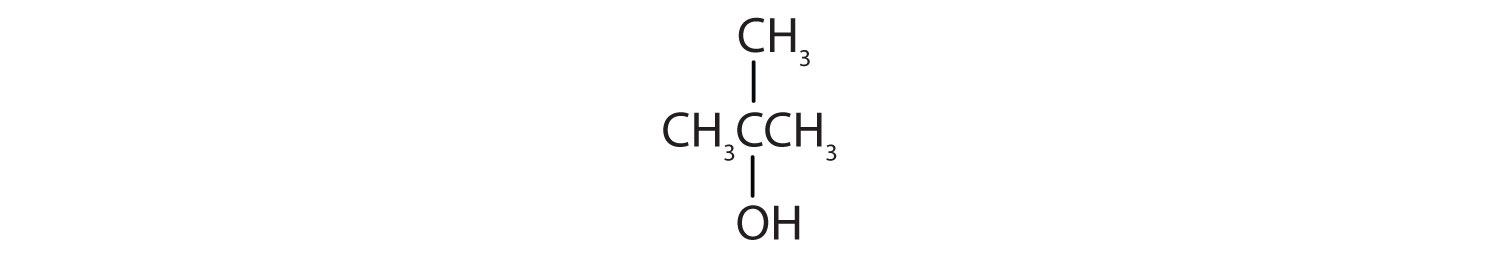
c.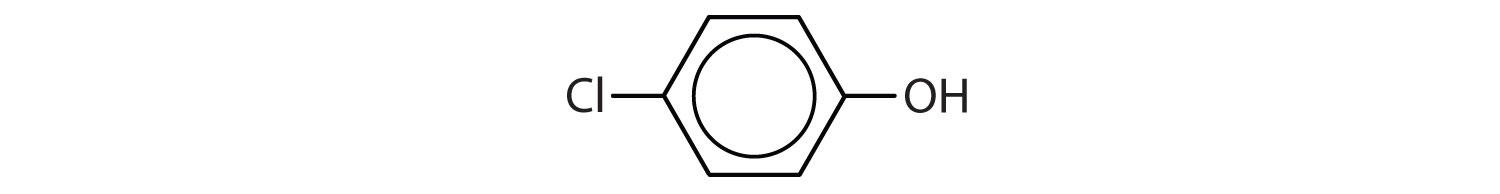
d.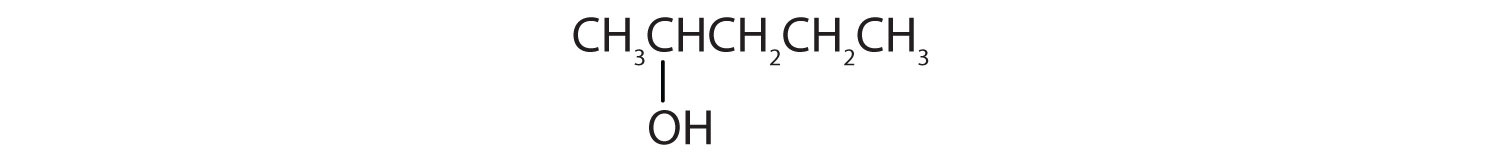
18. Tell whether each compound forms an acidic, a basic, or a neutral solution in water.
a.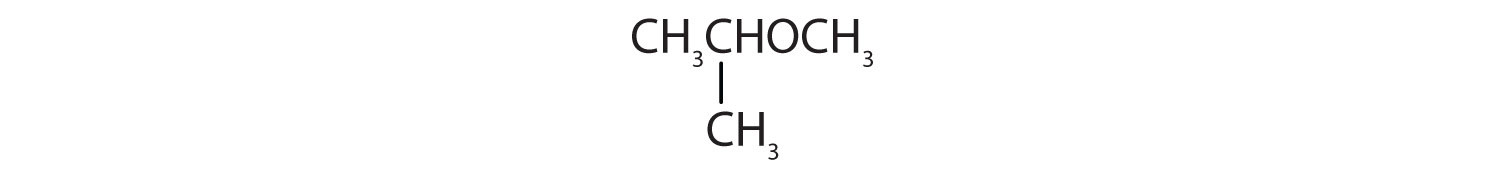
b.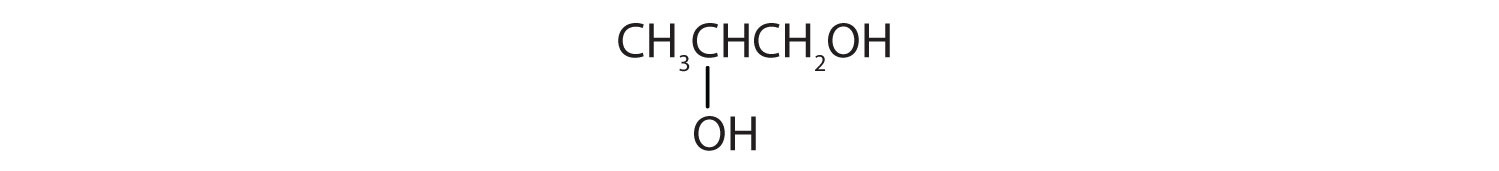
c.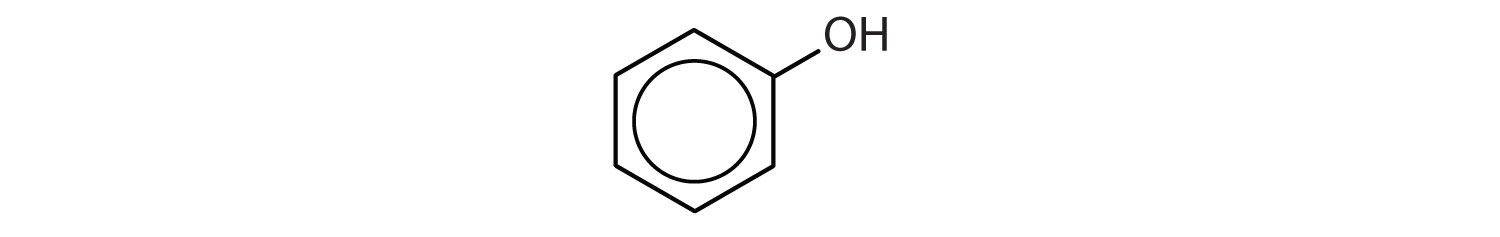
19. When water is added to ethylene in the presence of an acid catalyst, only one product—ethanol—is possible. However, when water is added to propylene, two products are possible—1-propanol and 2-propanol—but only 2-propanol is formed. In 1870, the Russian chemist Vladimir V. Markovnikov proposed a rule to predict the products of such reactions: Considering water to be HOH, the hydrogen atom of water goes on the carbon atom (of the two involved in the double bond) that has the most hydrogen atoms already bonded to it. The OH group goes on the carbon atom with fewer hydrogen atoms. Use Markovnikov’s rule to predict the product of the addition of water to each compound.
a. 2-methylpropene
b. 1-butene
c. 2-methyl-1-pentene
d. 2-methyl-2-pentene
20. Ethyl alcohol, like rubbing alcohol (isopropyl alcohol), is often used for sponge baths. What property of alcohols makes them useful for this purpose?
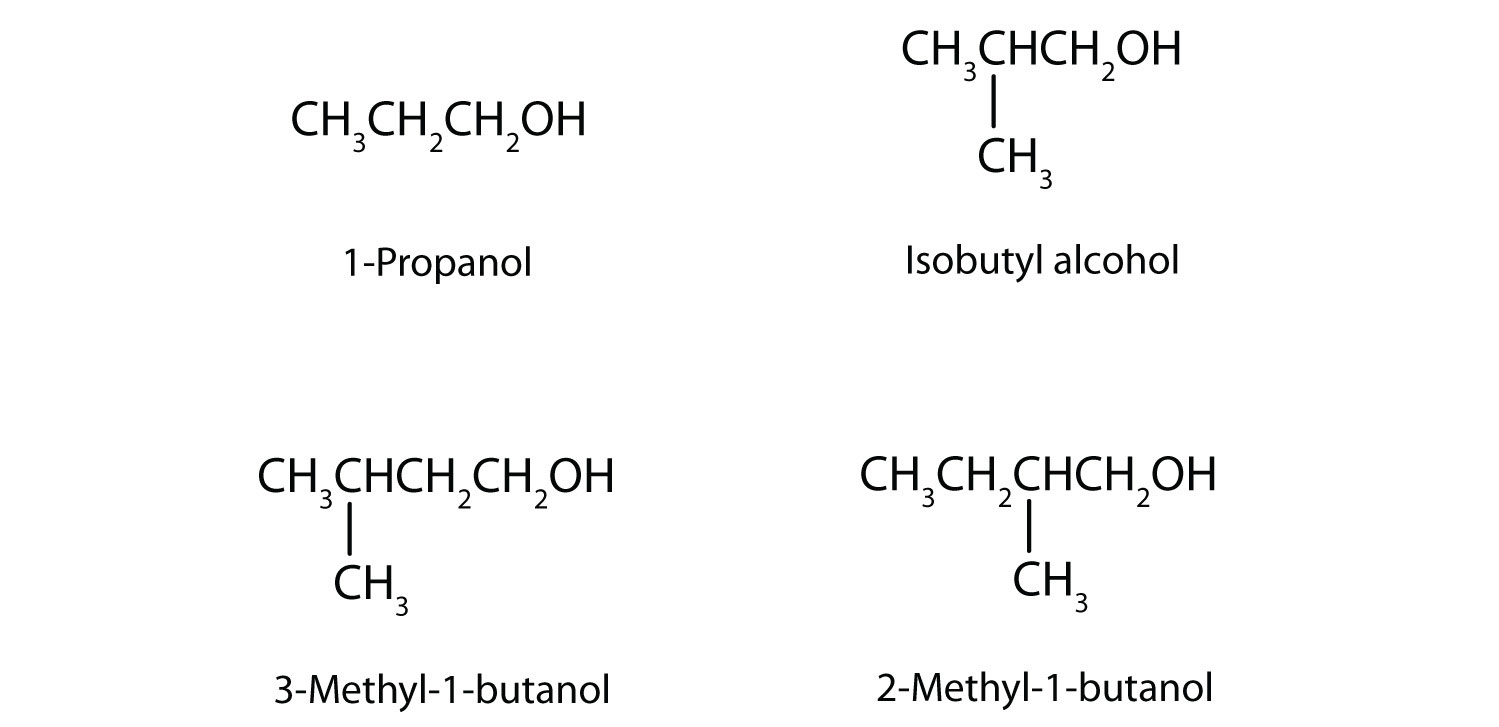
21. In addition to ethanol, the fermentation of grain produces other organic compounds collectively called fusel oils (FO). The four principal FO components are 1-propanol, isobutyl alcohol, 3-methyl-1-butanol, and 2-methyl-1-butanol. Draw a structure for each. (FO is quite toxic and accounts in part for hangovers.)
22. Draw and name the isomeric ethers that have the formula C5H12O.
23. Menthol is an ingredient in mentholated cough drops and nasal sprays. It produces a cooling, refreshing sensation when rubbed on the skin and so is used in shaving lotions and cosmetics. Thymol, the aromatic equivalent of menthol, is the flavoring constituent of thyme.
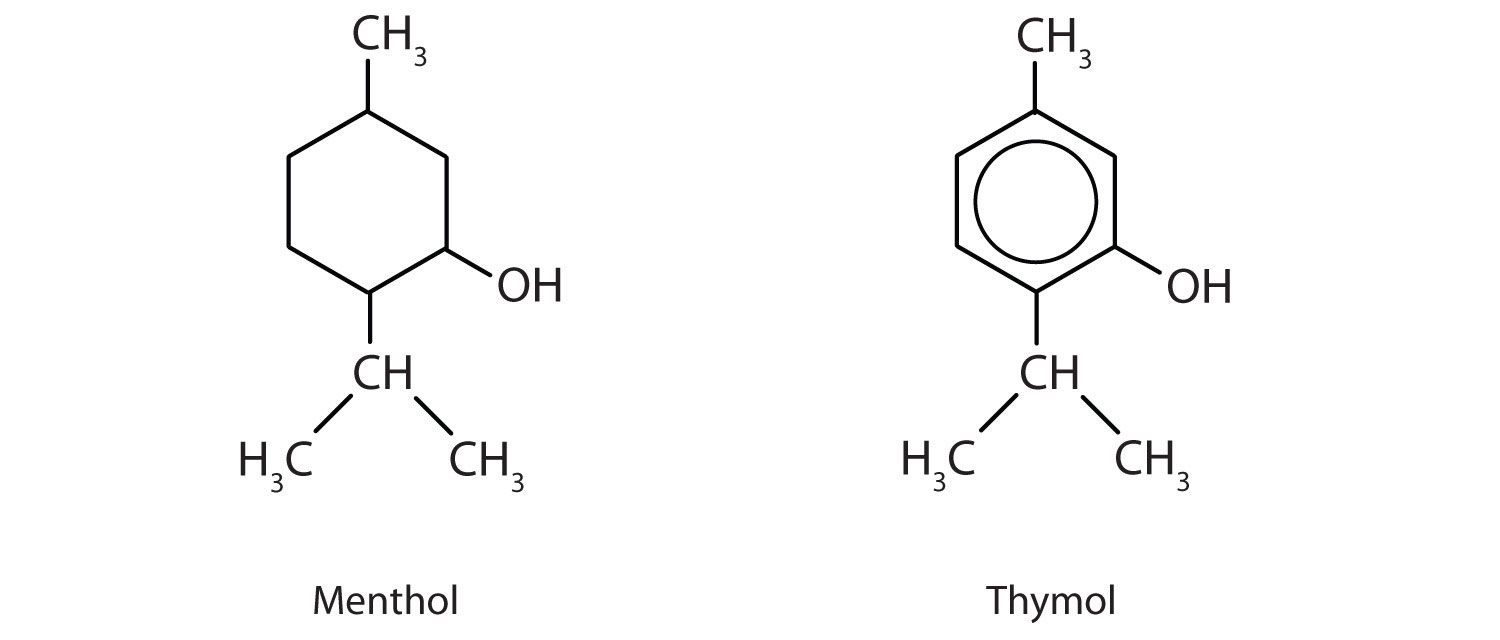
a. To what class of compounds does each belong?
b. Give an alternate name for thymol.
24. Write the equation for the production of ethanol by the addition of water to ethylene. How much ethanol can be made from 14.0 kg of ethylene?
25. Methanol is not particularly toxic to rats. If methanol were newly discovered and tested for toxicity in laboratory animals, what would you conclude about its safety for human consumption?
26. The amino acid cysteine has the formula HSCH2CH(NH2)COOH. What is the sulfur-containing functional group in the cysteine molecule?
27. The amino acid methionine has the formula CH3SCH2CH2CH(NH2)COOH. What functional groups are in methionine?
28. Tetrahydrocannabinol is the principal active ingredient in marijuana. What functional groups are present in this molecule?
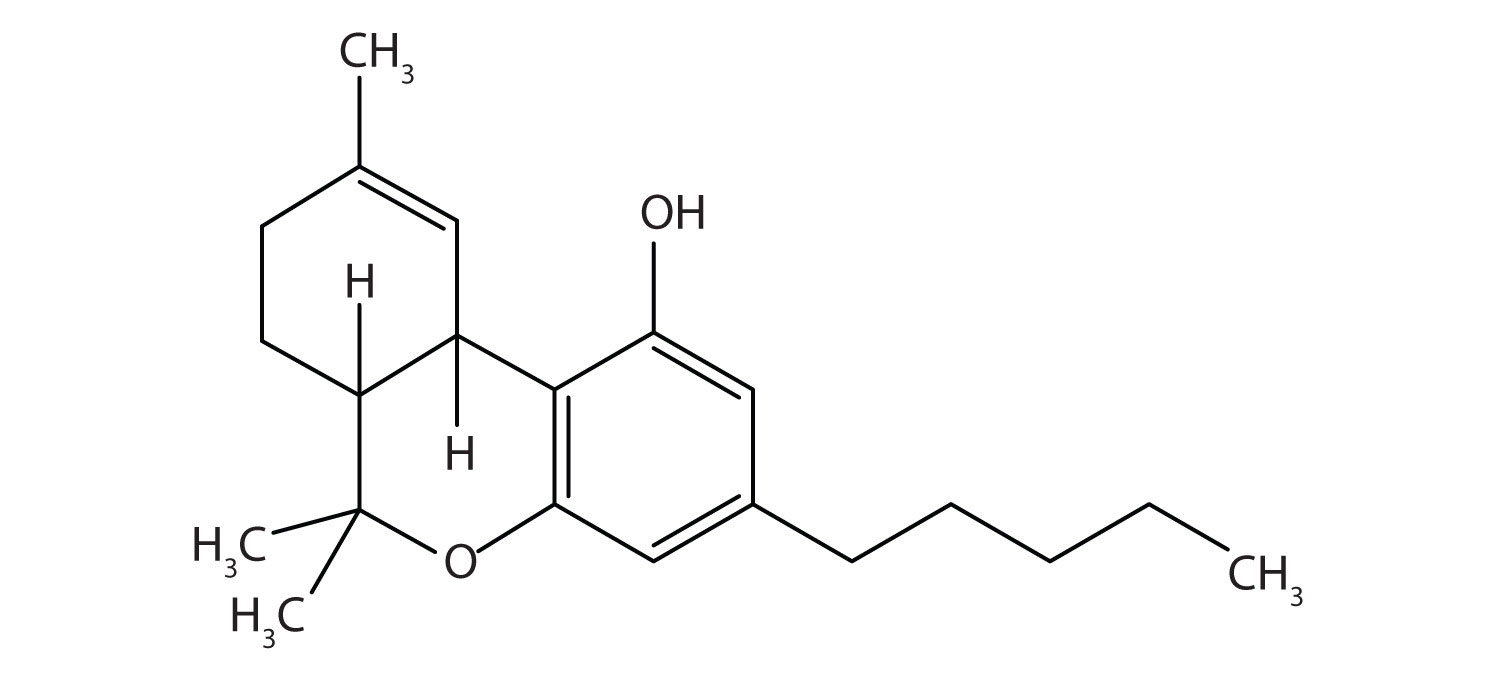
1. addition of water to ethylene; fermentation (for beverages)
3. Methanol is too toxic.
5.
a. oxidation
b. dehydration
c. hydration
7. Methanol is oxidized in the body to toxic formaldehyde; ethanol is oxidized to the less toxic acetaldehyde.
9.
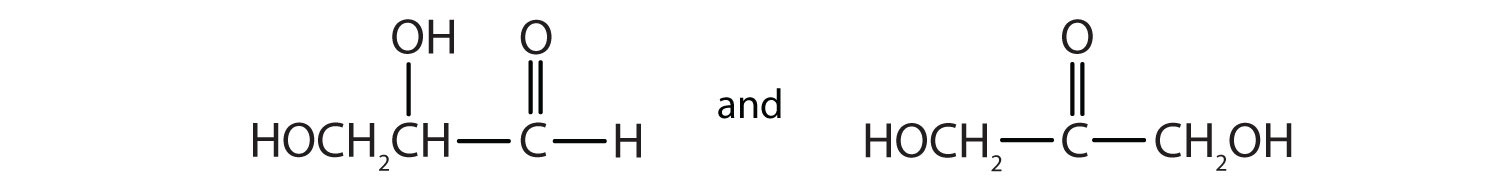
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
