Adapted by Nelson Nuñez-Rodriguez
Conditions of Use:

Unless otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Chapters derived from:
By David W. Ball
 Attribution-NonCommercial-ShareAlike
Attribution-NonCommercial-ShareAlike
CC BY-NC-SA
Click on the printer icon at the bottom of the screen
![]()
Make sure that your printout includes all content from the page. If it doesn't, try opening this guide in a different browser and printing from there (sometimes Internet Explorer works better, sometimes Chrome, sometimes Firefox, etc.).
If the above process produces printouts with errors or overlapping text or images, try this method:
Opening EssayPerhaps one of the most spectacular chemical reactions involving a gas occurred on May 6, 1937, when the German airship Hindenburg exploded on approach to the Naval Air Station in Lakehurst, New Jersey. The actual cause of the explosion is still unknown, but the entire volume of hydrogen gas used to float the airship, about 200,000 m3, burned in less than a minute. Thirty-six people, including one on the ground, were killed. Hydrogen is the lightest known gas. Any balloon filled with hydrogen gas will float in air if its mass is not too great. This makes hydrogen an obvious choice for flying machines based on balloons—airships, dirigibles, and blimps. However, hydrogen also has one obvious drawback: it burns in air according to the well-known chemical equation 2H2(g) + O2(g) → 2H2O(ℓ)So although hydrogen is an obvious choice, it is also a dangerous choice. Helium gas is also lighter than air and has 92% of the lifting power of hydrogen. Why, then, was helium not used in the Hindenburg? In the 1930s, helium was much more expensive. In addition, the best source of helium at the time was the United States, which banned helium exports to pre–World War II Germany. Today all airships use helium, a legacy of the Hindenburg disaster. |
Of the three basic phases of matter—solids, liquids, and gases—only one of them has predictable physical properties: gases. In fact, the study of the properties of gases was the beginning of the development of modern chemistry from its alchemical roots. The interesting thing about some of these properties is that they are independent of the identity of the gas. That is, it doesn’t matter if the gas is helium gas, oxygen gas, or sulfur vapors; some of their behavior is predictable and, as we will find, very similar. In this chapter, we will review some of the common behaviors of gases.
Let us start by reviewing some properties of gases. Gases have no definite shape or volume; they tend to fill whatever container they are in. They can compress and expand, sometimes to a great extent. Gases have extremely low densities, one-thousandth or less the density of a liquid or solid. Combinations of gases tend to mix together spontaneously; that is, they form solutions. Air, for example, is a solution of mostly nitrogen and oxygen. Any understanding of the properties of gases must be able to explain these characteristics.
Learning Objectives |
|
Gases were among the first substances studied in terms of the modern scientific method, which was developed in the 1600s. It did not take long to recognize that gases all shared certain physical behaviors, suggesting that all gases could be described by one all-encompassing theory. Today, that theory is the kinectic theory of gases. It is based on the following statements:
Figure 9.1 "The Kinetic Theory of Gases" shows a representation of how we mentally picture the gas phase.
Figure 9.1 The Kinetic Theory of Gases
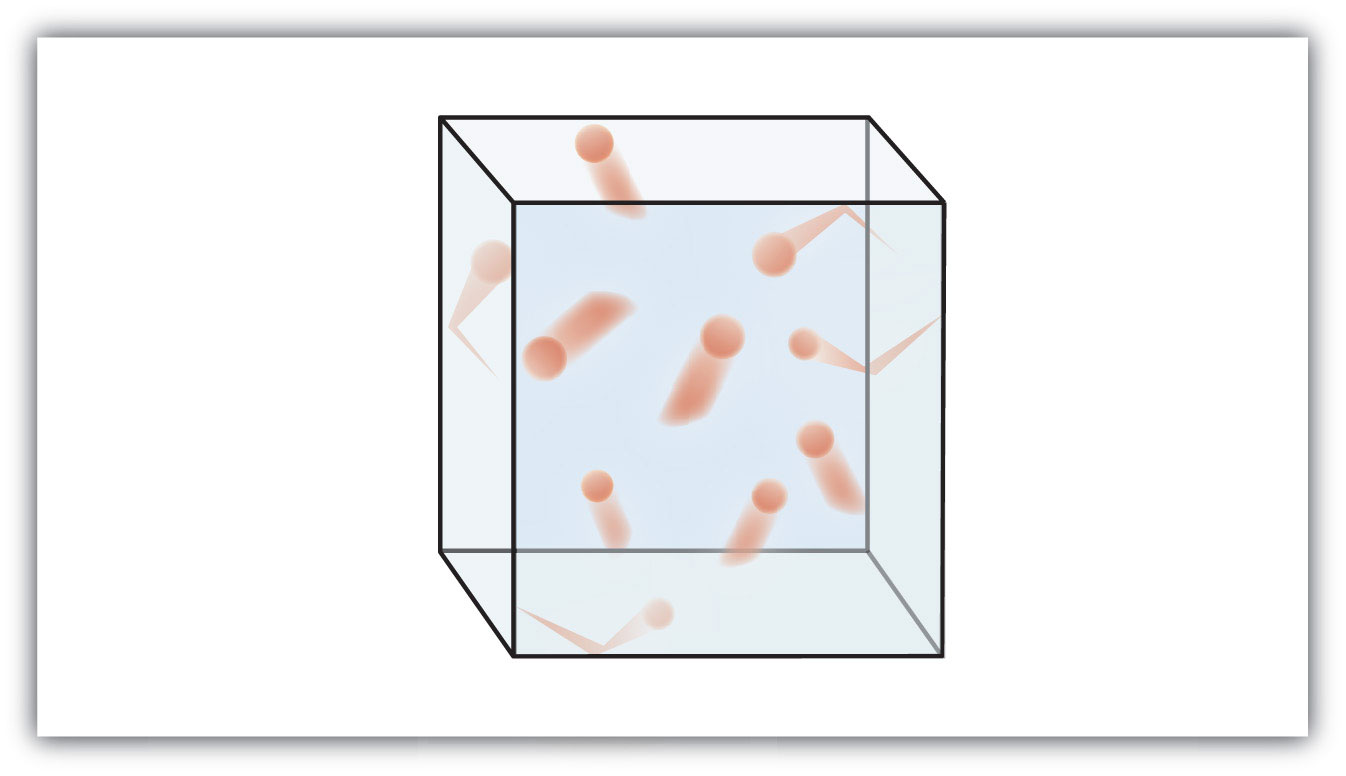
The kinetic theory of gases describes this state of matter as composed of tiny particles in constant motion with a lot of distance between the particles.
This model of gases explains some of the physical properties of gases. Because most of a gas is empty space, a gas has a low density and can expand or contract under the appropriate influence. The fact that gas particles are in constant motion means that two or more gases will always mix, as the particles from the individual gases move and collide with each other.
An ideal gas is a gas that exactly follows the statements of the kinetic theory. Unfortunately, real gases are not ideal. Many gases deviate slightly from agreeing perfectly with the kinetic theory of gases. However, most gases adhere to the statements so well that the kinetic theory of gases is well accepted by the scientific community.
Key Takeaways |
|
Exercises |
|
Learning Objectives |
|
The kinetic theory of gases indicates that gas particles are always in motion and are colliding with other particles and the walls of the container holding them. Although collisions with container walls are elastic (i.e., there is no net energy gain or loss because of the collision), a gas particle does exert a force on the wall during the collision. The accumulation of all these forces distributed over the area of the walls of the container causes something we call pressure. Pressure (P) is defined as the force of all the gas particle/wall collisions divided by the area of the wall:
All gases exert pressure; it is one of the fundamental measurable quantities of this phase of matter. Even our atmosphere exerts pressure—in this case, the gas is being “held in” by the earth’s gravity, rather than the gas being in a container. The pressure of the atmosphere is about 14.7 pounds of force for every square inch of surface area: 14.7 lb/in2.
Pressure has a variety of units. The formal, SI-approved unit of pressure is the pascal (Pa), which is defined as 1 N/m2 (one newton of force over an area of one square meter). However, this is usually too small in magnitude to be useful. A common unit of pressure is the atmosphere (atm), which was originally defined as the average atmospheric pressure at sea level.
However, “average atmospheric pressure at sea level” is difficult to pinpoint because of atmospheric pressure variations. A more reliable and common unit is millimeters of mercury (mmHg), which is the amount of pressure exerted by a column of mercury exactly 1 mm high. An equivalent unit is the torr , which equals 1 mmHg. (The torr is named after Evangelista Torricelli, a seventeenth-century Italian scientist who invented the mercury barometer.) With these definitions of pressure, the atmosphere unit is redefined: 1 atm is defined as exactly 760 mmHg, or 760 torr. We thus have the following equivalences:
1 atm = 760 mmHg = 760 torr
We can use these equivalences as with any equivalences—to perform conversions from one unit to another. Relating these to the formal SI unit of pressure, 1 atm = 101,325 Pa.
Example 1 |
|
How many atmospheres are there in 595 torr? Solution Using the pressure equivalences, we construct a conversion factor between torr and atmospheres: thus
Because the numbers in the conversion factor are exact, the number of significant figures in the final answer is determined by the initial value of pressure. Test Yourself How many atmospheres are there in 1,022 torr? Answer 1.345 atm |
Example 2 |
|
The atmosphere on Mars is largely CO2 at a pressure of 6.01 mmHg. What is this pressure in atmospheres? Solution Use the pressure equivalences to construct the proper conversion factor between millimeters of mercury and atmospheres.
At the end, we expressed the answer in scientific notation. Test Yourself Atmospheric pressure is low in the eye of a hurricane. In a 1979 hurricane in the Pacific Ocean, a pressure of 0.859 atm was reported inside the eye. What is this pressure in torr? Answer 652 torr |
Key Takeaways |
|
Exercises |
|
Learning Objectives |
|
When seventeenth-century scientists began studying the physical properties of gases, they noticed some simple relationships between some of the measurable properties of the gas. Take pressure (P) and volume (V), for example. Scientists noted that for a given amount of a gas (usually expressed in units of moles [n]), if the temperature (T) of the gas was kept constant, pressure and volume were related: As one increases, the other decreases. As one decreases, the other increases. We say that pressure and volume are inversely related.
There is more to it, however: pressure and volume of a given amount of gas at constant temperature are numerically related. If you take the pressure value and multiply it by the volume value, the product is a constant for a given amount of gas at a constant temperature:
P × V = constant at constant n and T
If either volume or pressure changes while amount and temperature stay the same, then the other property must change so that the product of the two properties still equals that same constant. That is, if the original conditions are labeled P1 and V1 and the new conditions are labeled P2 and V2, we have
P1V1 = constant = P2V2
where the properties are assumed to be multiplied together. Leaving out the middle part, we have simply
P1V1 = P2V2 at constant n and T
This equation is an example of a gas law. A gas law is a simple mathematical formula that allows you to model, or predict, the behavior of a gas. This particular gas law is called Boyle's law, after the English scientist Robert Boyle, who first announced it in 1662. Figure 9.2 "Boyle’s Law" shows two representations of how Boyle’s law works.
Figure 9.2 Boyle’s Law
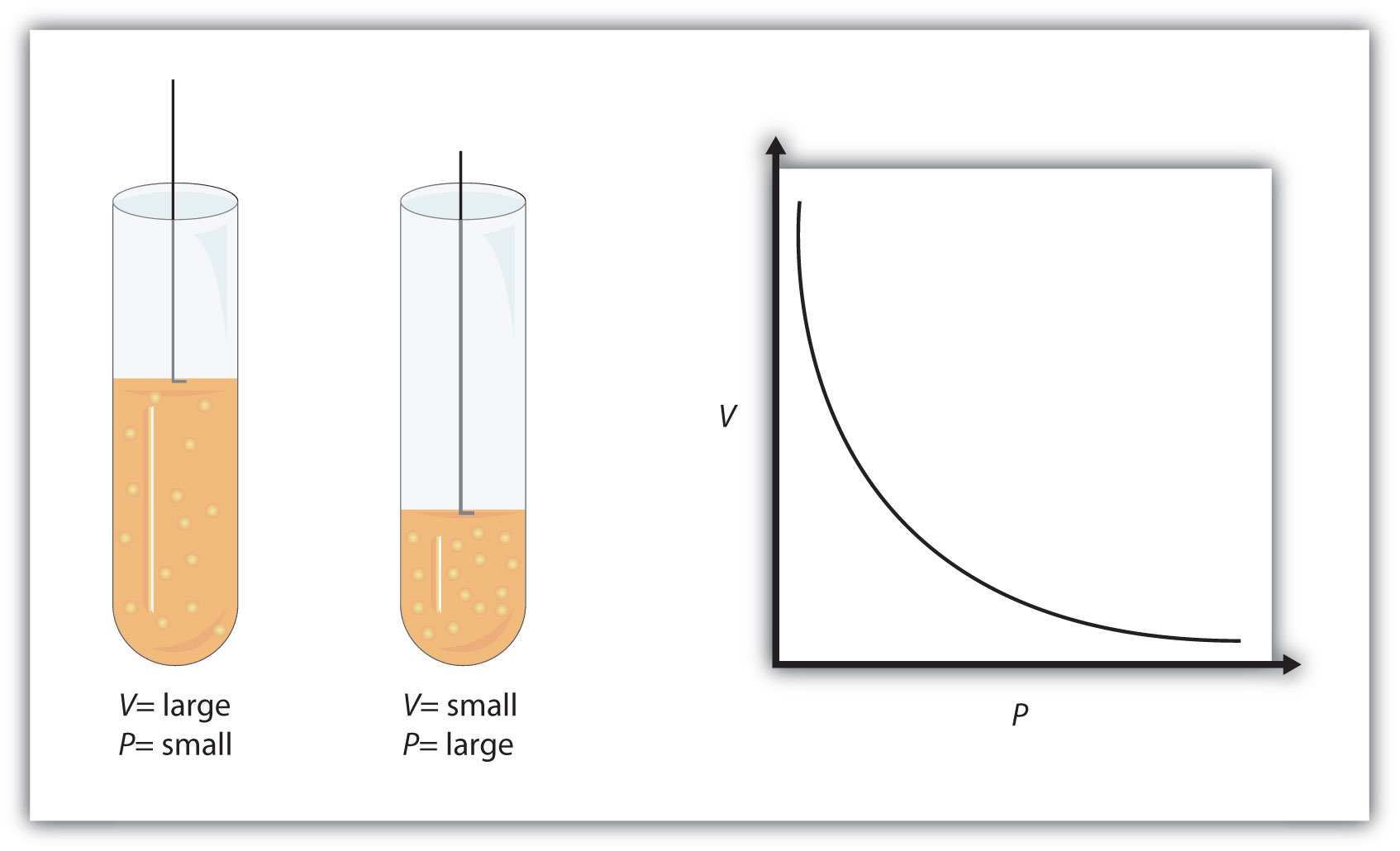
A piston having a certain pressure and volume (left piston) will have half the volume when its pressure is twice as much (right piston). One can also plot P versus V for a given amount of gas at a certain temperature; such a plot will look like the graph on the right.
Boyle’s law is an example of a second type of mathematical problem we see in chemistry—one based on a mathematical formula. Tactics for working with mathematical formulas are different from tactics for working with conversion factors. First, most of the questions you will have to answer using formulas are word-type questions, so the first step is to identify what quantities are known and assign them to variables. Second, in most formulas, some mathematical rearrangements (i.e., algebra) must be performed to solve for an unknown variable. The rule is that to find the value of the unknown variable, you must mathematically isolate the unknown variable by itself and in the numerator of one side of the equation. Finally, units must be consistent. For example, in Boyle’s law there are two pressure variables; they must have the same unit. There are also two volume variables; they also must have the same unit. In most cases, it won’t matter what the unit is, but the unit must be the same on both sides of the equation.
Example 3 |
|
A sample of gas has an initial pressure of 2.44 atm and an initial volume of 4.01 L. Its pressure changes to 1.93 atm. What is the new volume if temperature and amount are kept constant? Solution First, determine what quantities we are given. We are given an initial pressure and an initial volume, so let these values be P1 and V1: P1 = 2.44 atm and V1 = 4.01 L
We are given another quantity, final pressure of 1.93 atm, but not a final volume. This final volume is the variable we will solve for. P2 = 1.93 atm and V2 = ? L
Substituting these values into Boyle’s law, we get (2.44 atm)(4.01 L) = (1.93 atm)V2
To solve for the unknown variable, we isolate it by dividing both sides of the equation by 1.93 atm—both the number and the unit:
Note that, on the left side of the equation, the unit atm is in the numerator and the denominator of the fraction. They cancel algebraically, just as a number would. On the right side, the unit atm and the number 1.93 are in the numerator and the denominator, so the entire quantity cancels:
What we have left is
Now we simply multiply and divide the numbers together and combine the answer with the L unit, which is a unit of volume. Doing so, we get V2 = 5.07 L
Does this answer make sense? We know that pressure and volume are inversely related; as one decreases, the other increases. Pressure is decreasing (from 2.44 atm to 1.93 atm), so volume should be increasing to compensate, and it is (from 4.01 L to 5.07 L). So the answer makes sense based on Boyle’s law. Test Yourself If P1 = 334 torr, V1 = 37.8 mL, and P2 = 102 torr, what is V2? Answer 124 mL |
As mentioned, you can use any units for pressure or volume, but both pressures must be expressed in the same units, and both volumes must be expressed in the same units.
Example 4 |
|
A sample of gas has an initial pressure of 722 torr and an initial volume of 88.8 mL. Its volume changes to 0.663 L. What is the new pressure? Solution We can still use Boyle’s law to answer this, but now the two volume quantities have different units. It does not matter which unit we change, as long as we perform the conversion correctly. Let us change the 0.663 L to milliliters:
Now that both volume quantities have the same units, we can substitute into Boyle’s law:
The mL units cancel, and we multiply and divide the numbers to get P2 = 96.7 torr The volume is increasing, and the pressure is decreasing, which is as expected for Boyle’s law.
Test Yourself If V1 = 456 mL, P1 = 308 torr, and P2 = 1.55 atm, what is V2?
Answer 119 mL |
There are other measurable characteristics of a gas. One of them is temperature (T). Perhaps one can vary the temperature of a gas sample and note what effect it has on the other properties of the gas. Early scientists did just this, discovering that if the amount of a gas and its pressure are kept constant, then changing the temperature changes the volume (V). As temperature increases, volume increases; as temperature decreases, volume decreases. We say that these two characteristics are directly related.
A mathematical relationship between V and T should be possible except for one thought: what temperature scale should we use? We know from Chapter 1 "Measurements" that science uses several possible temperature scales. Experiments show that the volume of a gas is related to its absolute temperature in Kelvin, not its temperature in degrees Celsius. If the temperature of a gas is expressed in kelvins, then experiments show that the ratio of volume to temperature is a constant:
We can modify this equation as we modified Boyle’s law: the initial conditions V1 and T1 have a certain value, and the value must be the same when the conditions of the gas are changed to some new conditions V2 and T2, as long as pressure and the amount of the gas remain constant. Thus, we have another gas law:
This gas law is commonly referred to as Charles's law, after the French scientist Jacques Charles, who performed experiments on gases in the 1780s. The tactics for using this mathematical formula are similar to those for Boyle’s law. To determine an unknown quantity, use algebra to isolate the unknown variable by itself and in the numerator; the units of similar variables must be the same. But we add one more tactic: all temperatures must be expressed in the absolute temperature scale (Kelvin). As a reminder, we review the conversion between the absolute temperature scale and the Celsius temperature scale:
K = °C + 273
where K represents the temperature in kelvins, and °C represents the temperature in degrees Celsius.
Figure 9.3 "Charles’s Law" shows two representations of how Charles’s law works.
Figure 9.3 Charles’s Law
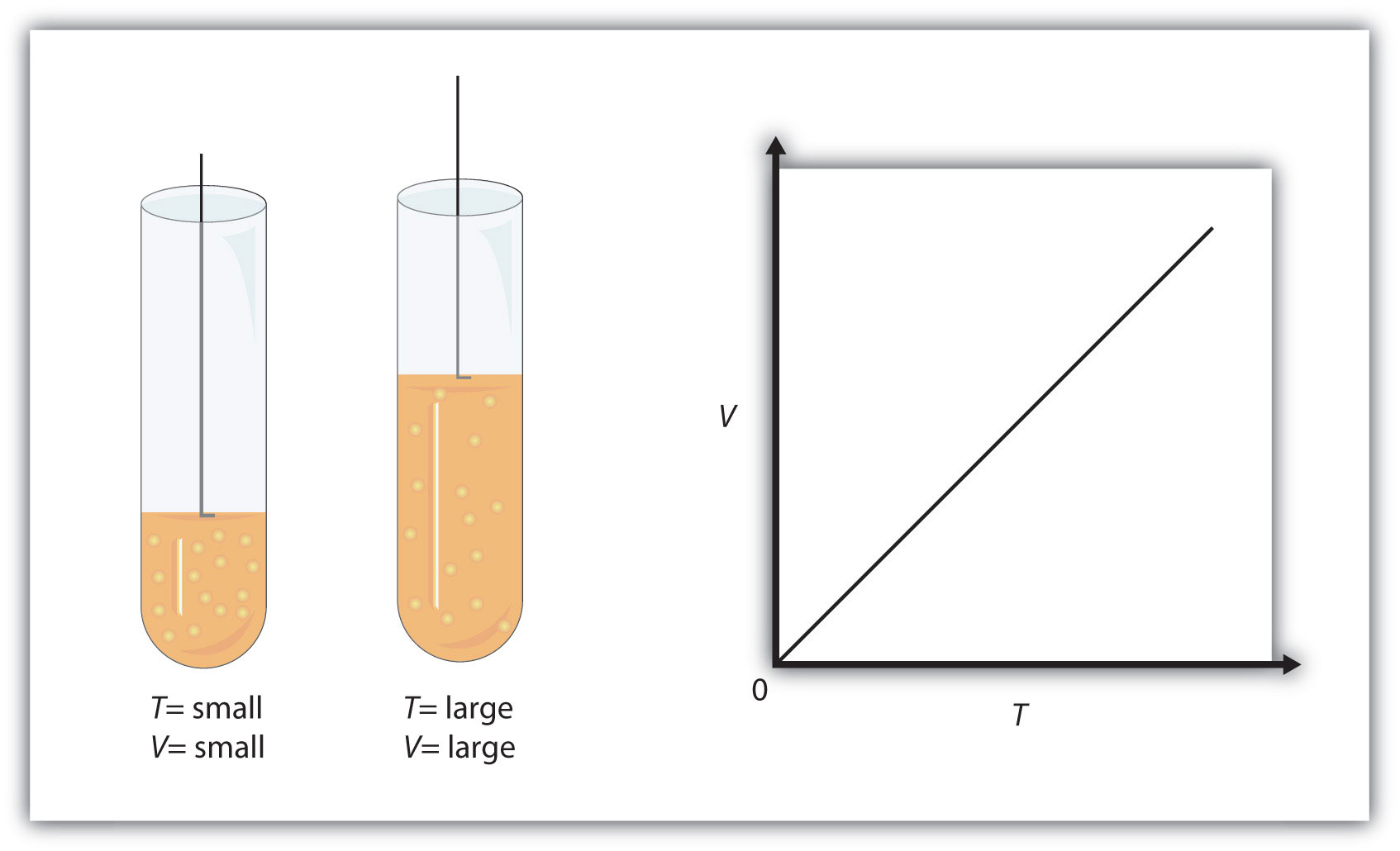
A piston having a certain volume and temperature (left piston) will have twice the volume when its temperature is twice as much (right piston). One can also plot V versus T for a given amount of gas at a certain pressure; such a plot will look like the graph on the right.
Example 5 |
|
A sample of gas has an initial volume of 34.8 mL and an initial temperature of 315 K. What is the new volume if the temperature is increased to 559 K? Assume constant pressure and amount for the gas. Solution First, we assign the given values to their variables. The initial volume is V1, so V1 = 34.8 mL, and the initial temperature is T1, so T1 = 315 K. The temperature is increased to 559 K, so the final temperature T2 = 559 K. We note that the temperatures are already given in kelvins, so we do not need to convert the temperatures. Substituting into the expression for Charles’s law yields
We solve for V2 by algebraically isolating the V2 variable on one side of the equation. We do this by multiplying both sides of the equation by 559 K (number and unit). When we do this, the temperature unit cancels on the left side, while the entire 559 K cancels on the right side:
The expression simplifies to
By multiplying and dividing the numbers, we see that the only remaining unit is mL, so our final answer is V2 = 61.8 mL
Does this answer make sense? We know that as temperature increases, volume increases. Here, the temperature is increasing from 315 K to 559 K, so the volume should also increase, which it does. Test Yourself If V1 = 3.77 L and T1 = 255 K, what is V2 if T2 = 123 K? Answer 1.82 L
|
It is more mathematically complicated if a final temperature must be calculated because the T variable is in the denominator of Charles’s law. There are several mathematical ways to work this, but perhaps the simplest way is to take the reciprocal of Charles’s law. That is, rather than write it as
write the equation as
It is still an equality and a correct form of Charles’s law, but now the temperature variable is in the numerator, and the algebra required to predict a final temperature is simpler.
Example 6 |
|
A sample of a gas has an initial volume of 34.8 L and an initial temperature of −67°C. What must be the temperature of the gas for its volume to be 25.0 L? Solution Here, we are looking for a final temperature, so we will use the reciprocal form of Charles’s law. However, the initial temperature is given in degrees Celsius, not kelvins. We must convert the initial temperature to kelvins: −67°C + 273 = 206 K
In using the gas law, we must use T1 = 206 K as the temperature. Substituting into the reciprocal form of Charles’s law, we get
Bringing the 25.0 L quantity over to the other side of the equation, we get
The L units cancel, so our final answer is T2 = 148 K
This is also equal to −125°C. As temperature decreases, volume decreases, which it does in this example.
Test Yourself If V1 = 623 mL, T1 = 255°C, and V2 = 277 mL, what is T2?
Answer 235 K, or −38°C |
Key Takeaways |
|
Exercises |
|
Learning Objectives |
|
You may notice in Boyle’s law and Charles’s law that we actually refer to four physical properties of a gas: pressure (P), volume (V), temperature (T), and amount (in moles; n). We do this because these are the only four independent physical properties of a gas. There are other physical properties, but they are all related to one (or more) of these four properties.
Boyle’s law is written in terms of two of these properties, with the other two being held constant. Charles’s law is written in terms of two different properties, with the other two being held constant. It may not be surprising to learn that there are other gas laws that relate other pairs of properties—as long as the other two are held constant. Here we will mention a few.
Gay-Lussac’s law relates pressure with absolute temperature. In terms of two sets of data, Gay-Lussac’s law is
Note that it has a structure very similar to that of Charles’s law, only with different variables—pressure instead of volume. Avogadro’s law introduces the last variable for amount. The original statement of Avogadro’s law states that equal volumes of different gases at the same temperature and pressure contain the same number of particles of gas. Because the number of particles is related to the number of moles (1 mol = 6.022 × 1023 particles), Avogadro’s law essentially states that equal volumes of different gases at the same temperature and pressure contain the same amount (moles, particles) of gas. Put mathematically into a gas law, Avogadro’s law is
(First announced in 1811, it was Avogadro’s proposal that volume is related to the number of particles that eventually led to naming the number of things in a mole as Avogadro’s number.) Avogadro’s law is useful because for the first time we are seeing amount, in terms of the number of moles, as a variable in a gas law.
Example 7 |
|
A 2.45 L volume of gas contains 4.5 × 1021 gas particles. How many gas particles are there in 3.87 L if the gas is at constant pressure and temperature? Solution We can set up Avogadro’s law as follows:
We algebraically rearrange to solve for n2:
The L units cancel, so we solve for n2: n2 = 7.1 × 1021 particles
Test Yourself A 12.8 L volume of gas contains 3.00 × 1020 gas particles. At constant temperature and pressure, what volume does 8.22 × 1018 gas particles fill? Answer 0.351 L |
The variable n in Avogadro’s law can also stand for the number of moles of gas in addition to number of particles.
One thing we notice about all the gas laws is that, collectively, volume and pressure are always in the numerator, and temperature is always in the denominator. This suggests that we can propose a gas law that combines pressure, volume, and temperature. This gas law is known as the combined gas law, and its mathematical form is
This allows us to follow changes in all three major properties of a gas. Again, the usual warnings apply about how to solve for an unknown algebraically (isolate it on one side of the equation in the numerator), units (they must be the same for the two similar variables of each type), and units of temperature must be in kelvins.
Example 8 |
|
A sample of gas at an initial volume of 8.33 L, an initial pressure of 1.82 atm, and an initial temperature of 286 K simultaneously changes its temperature to 355 K and its volume to 5.72 L. What is the final pressure of the gas? Solution We can use the combined gas law directly; all the units are consistent with each other, and the temperatures are given in Kelvin. Substituting,
We rearrange this to isolate the P2 variable all by itself. When we do so, certain units cancel:
Multiplying and dividing all the numbers, we get P2 = 3.29 atm
Ultimately, the pressure increased, which would have been difficult to predict because two properties of the gas were changing. Test Yourself If P1 = 662 torr, V1 = 46.7 mL, T1 = 266 K, P2 = 409 torr, and T2 = 371 K, what is V2? Answer 105 mL |
As with other gas laws, if you need to determine the value of a variable in the denominator of the combined gas law, you can either cross-multiply all the terms or just take the reciprocal of the combined gas law. Remember, the variable you are solving for must be in the numerator and all by itself on one side of the equation.
Key Takeaways |
|
Exercises |
||||||||||||||||||||||||||||||||||||||||||||||||
|
Learning Objectives |
|
So far, the gas laws we have considered have all required that the gas change its conditions; then we predict a resulting change in one of its properties. Are there any gas laws that relate the physical properties of a gas at any given time?
Consider a further extension of the combined gas law to include n. By analogy to Avogadro’s law, n is positioned in the denominator of the fraction, opposite the volume. So
Because pressure, volume, temperature, and amount are the only four independent physical properties of a gas, the constant in the above equation is truly a constant; indeed, because we do not need to specify the identity of a gas to apply the gas laws, this constant is the same for all gases. We define this constant with the symbol R, so the previous equation is written as
which is usually rearranged as
PV = nRT
This equation is called the ideal gas law It relates the four independent properties of a gas at any time. The constant R is called the ideal gas law constant. Its value depends on the units used to express pressure and volume. Table 9.1 "Values of the Ideal Gas Law Constant " lists the numerical values of R.
Table 9.1 Values of the Ideal Gas Law Constant R
| Numerical Value | Units |
|---|---|
| 0.08205 | |
| 62.36 | |
| 8.314 |
The ideal gas law is used like any other gas law, with attention paid to the unit and making sure that temperature is expressed in Kelvin. However, the ideal gas law does not require a change in the conditions of a gas sample. The ideal gas law implies that if you know any three of the physical properties of a gas, you can calculate the fourth property.
Example 9 |
|
A 4.22 mol sample of Ar has a pressure of 1.21 atm and a temperature of 34°C. What is its volume? Solution The first step is to convert temperature to kelvins: 34 + 273 = 307 K
Now we can substitute the conditions into the ideal gas law:
The atm unit is in the numerator of both sides, so it cancels. On the right side of the equation, the mol and K units appear in the numerator and the denominator, so they cancel as well. The only unit remaining is L, which is the unit of volume that we are looking for. We isolate the volume variable by dividing both sides of the equation by 1.21:
Then solving for volume, we get V = 87.9 L
Test Yourself A 0.0997 mol sample of O2 has a pressure of 0.692 atm and a temperature of 333 K. What is its volume? Answer 3.94 L |
Example 10 |
|
At a given temperature, 0.00332 g of Hg in the gas phase has a pressure of 0.00120 mmHg and a volume of 435 L. What is its temperature? Solution We are not given the number of moles of Hg directly, but we are given a mass. We can use the molar mass of Hg to convert to the number of moles.
Pressure is given in units of millimeters of mercury. We can either convert this to atmospheres or use the value of the ideal gas constant that includes the mmHg unit. We will take the second option. Substituting into the ideal gas law,
The mmHg, L, and mol units cancel, leaving the K unit, the unit of temperature. Isolating T all by itself on one side, we get
Then solving for K, we get T = 1,404 K
Test Yourself For a 0.00554 mol sample of H2, P = 23.44 torr and T = 557 K. What is its volume? Answer 8.21 L |
The ideal gas law can also be used in stoichiometry problems.
Example 11 |
|
What volume of H2 is produced at 299 K and 1.07 atm when 55.8 g of Zn metal react with excess HCl? Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Solution Here we have a stoichiometry problem where we need to find the number of moles of H2 produced. Then we can use the ideal gas law, with the given temperature and pressure, to determine the volume of gas produced. First, the number of moles of H2 is calculated: Now that we know the number of moles of gas, we can use the ideal gas law to determine the volume, given the other conditions: All the units cancel except for L, for volume, which means V = 19.6 L
Test Yourself What pressure of HCl is generated if 3.44 g of Cl2 are reacted in 4.55 L at 455 K? H2(g) + Cl2(g) → 2HCl(g)
Answer 0.796 atm |
It should be obvious by now that some physical properties of gases depend strongly on the conditions. What we need is a set of standard conditions so that properties of gases can be properly compared to each other. Standard temperature and pressure (STP) is defined as exactly 100 kPa of pressure (0.986 atm) and 273 K (0°C). For simplicity, we will use 1 atm as standard pressure. Defining STP allows us to compare more directly the properties of gases that differ from each other.
One property shared among gases is a molar volume. The molar volume is the volume of 1 mol of a gas. At STP, the molar volume of a gas can be easily determined by using the ideal gas law:
All the units cancel except for L, the unit of volume. So
V = 22.4 L
Note that we have not specified the identity of the gas; we have specified only that the pressure is 1 atm and the temperature is 273 K. This makes for a very useful approximation: any gas at STP has a volume of 22.4 L per mole of gas; that is, the molar volume at STP is 22.4 L/mol (Figure 9.4 "Molar Volume"). This molar volume makes a useful conversion factor in stoichiometry problems if the conditions are at STP. If the conditions are not at STP, a molar volume of 22.4 L/mol is not applicable. However, if the conditions are not at STP, the combined gas law can be used to calculate what the volume of the gas would be if at STP; then the 22.4 L/mol molar volume can be used.
Figure 9.4 Molar Volume
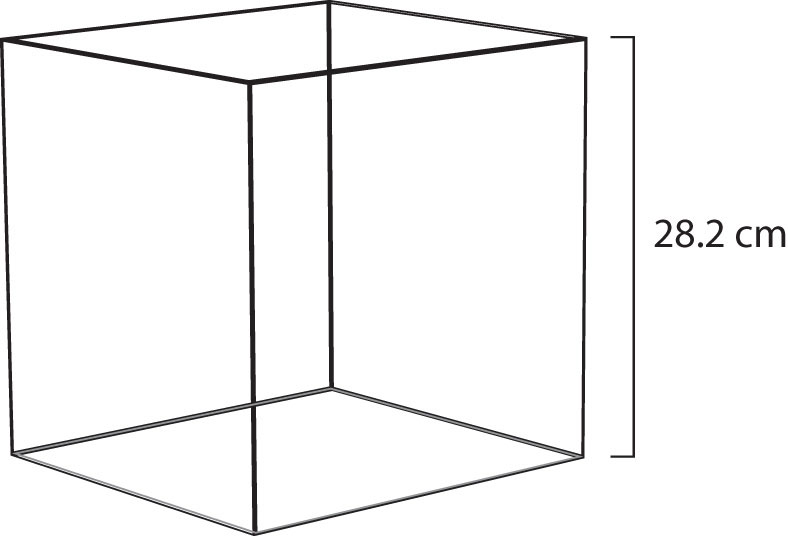
A mole of gas at STP occupies 22.4 L, the volume of a cube that is 28.2 cm on a side.
Example 12 |
|
How many moles of Ar are present in 38.7 L at STP? Solution We can use the molar volume, 22.4 L/mol, as a conversion factor, but we need to reverse the fraction so that the L units cancel and mol units are introduced. It is a one-step conversion: Test Yourself What volume does 4.87 mol of Kr have at STP? Answer 109 L |
Example 13 |
|
What volume of H2 is produced at STP when 55.8 g of Zn metal react with excess HCl? Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Solution This is a stoichiometry problem with a twist: we need to use the molar volume of a gas at STP to determine the final answer. The first part of the calculation is the same as in a previous example:
Now we can use the molar volume, 22.4 L/mol, because the gas is at STP:
Alternatively, we could have applied the molar volume as a third conversion factor in the original stoichiometry calculation. Test Yourself What volume of HCl is generated if 3.44 g of Cl2 are reacted at STP? H2(g) + Cl2(g) → 2HCl(g)
Answer 2.17 L |
The ideal gas law can also be used to determine the densities of gases. Density, recall, is defined as the mass of a substance divided by its volume:
Assume that you have exactly 1 mol of a gas. If you know the identity of the gas, you can determine the molar mass of the substance. Using the ideal gas law, you can also determine the volume of that mole of gas, using whatever the temperature and pressure conditions are. Then you can calculate the density of the gas by using
Example 14 |
|
What is the density of N2 at 25°C and 0.955 atm? Solution First, we must convert the temperature into kelvins: 25 + 273 = 298 KIf we assume exactly 1 mol of N2, then we know its mass: 28.0 g. Using the ideal gas law, we can calculate the volume:
All the units cancel except for L, the unit of volume. So V = 25.6 L
Knowing the molar mass and the molar volume, we can determine the density of N2 under these conditions:
Test Yourself What is the density of CO2 at a pressure of 0.0079 atm and 227 K? (These are the approximate atmospheric conditions on Mars.) Answer 0.019 g/L |
Chemistry Is Everywhere: BreathingBreathing (more properly called respiration) is the process by which we draw air into our lungs so that our bodies can take up oxygen from the air. Let us apply the gas laws to breathing. Start by considering pressure. We draw air into our lungs because the diaphragm, a muscle underneath the lungs, moves down to reduce pressure in the lungs, causing external air to rush in to fill the lower-pressure volume. We expel air by the diaphragm pushing against the lungs, increasing pressure inside the lungs and forcing the high-pressure air out. What are the pressure changes involved? A quarter of an atmosphere? A tenth of an atmosphere? Actually, under normal conditions, it’s only 1 or 2 torr of pressure difference that makes us breathe in and out. Figure 9.5 Breathing Mechanics
Breathing involves pressure differences between the inside of the lungs and the air outside. The pressure differences are only a few torr. A normal breath is about 0.50 L. If room temperature is about 22°C, then the air has a temperature of about 295 K. With normal pressure being 1.0 atm, how many moles of air do we take in for every breath? The ideal gas law gives us an answer:
Solving for the number of moles, we get n = 0.021 mol airThis ends up being about 0.6 g of air per breath—not much but enough to keep us alive. |
Key Takeaways |
|
Exercises |
|
Learning Objective |
| Learn Dalton’s law of partial pressures. |
One of the properties of gases is that they mix with each other. When they do so, they become a solution—a homogeneous mixture. Some of the properties of gas mixtures are easy to determine if we know the composition of the gases in the mix.
In gas mixtures, each component in the gas phase can be treated separately. Each component of the mixture shares the same temperature and volume. (Remember that gases expand to fill the volume of their container; gases in a mixture continue to do that as well.) However, each gas has its own pressure. The partial pressure of a gas, Pi, is the pressure that an individual gas in a mixture has. Partial pressures are expressed in torr, millimeters of mercury, or atmospheres like any other gas pressure; however, we use the term pressure when talking about pure gases and the term partial pressure when we are talking about the individual gas components in a mixture.
Dalton’s law of partial pressures states that the total pressure of a gas mixture, Ptot, is equal to the sum of the partial pressures of the components, Pi:
Although this may seem to be a trivial law, it reinforces the idea that gases behave independently of each other.
Example 15 |
|
A mixture of H2 at 2.33 atm and N2 at 0.77 atm is in a container. What is the total pressure in the container? Solution Dalton’s law of partial pressures states that the total pressure is equal to the sum of the partial pressures. We simply add the two pressures together: Ptot = 2.33 atm + 0.77 atm = 3.10 atm
Test Yourself Air can be thought of as a mixture of N2 and O2. In 760 torr of air, the partial pressure of N2 is 608 torr. What is the partial pressure of O2? Answer 152 torr |
Example 16 |
|
A 2.00 L container with 2.50 atm of H2 is connected to a 5.00 L container with 1.90 atm of O2 inside. The containers are opened, and the gases mix. What is the final pressure inside the containers? Solution Because gases act independently of each other, we can determine the resulting final pressures using Boyle’s law and then add the two resulting pressures together to get the final pressure. The total final volume is 2.00 L + 5.00 L = 7.00 L. First, we use Boyle’s law to determine the final pressure of H2: (2.50 atm)(2.00 L) = P2(7.00 L)Solving for P2, we get P2 = 0.714 atm = partial pressure of H2Now we do that same thing for the O2: (1.90 atm)(5.00 L) = P2(7.00 L) P2 = 1.36 atm = partial pressure of O2The total pressure is the sum of the two resulting partial pressures: Ptot = 0.714 atm + 1.36 atm = 2.07 atm
Test Yourself If 0.75 atm of He in a 2.00 L container is connected to a 3.00 L container with 0.35 atm of Ne and the containers are opened, what is the resulting total pressure? Answer 0.51 atm |
One of the reasons we have to deal with Dalton’s law of partial pressures is because gases are frequently collected by bubbling through water. As we will see in Chapter 3 "Solids and Liquids", liquids are constantly evaporating into a vapor until the vapor achieves a partial pressure characteristic of the substance and the temperature. This partial pressure is called a vapor pressure. Table 9.2 "Vapor Pressure of Water versus Temperature" lists the vapor pressures of H2O versus temperature. Note that if a substance is normally a gas under a given set of conditions, the term partial pressure is used; the term vapor pressure is reserved for the partial pressure of a vapor when the liquid is the normal phase under a given set of conditions.
Table 9.2 Vapor Pressure of Water versus Temperature
| Temperature (°C) | Vapor Pressure (torr) | Temperature (°C) | Vapor Pressure (torr) | |
|---|---|---|---|---|
| 5 | 6.54 | 30 | 31.84 | |
| 10 | 9.21 | 35 | 42.20 | |
| 15 | 12.79 | 40 | 55.36 | |
| 20 | 17.54 | 50 | 92.59 | |
| 21 | 18.66 | 60 | 149.5 | |
| 22 | 19.84 | 70 | 233.8 | |
| 23 | 21.08 | 80 | 355.3 | |
| 24 | 22.39 | 90 | 525.9 | |
| 25 | 23.77 | 100 | 760.0 |
Any time a gas is collected over water, the total pressure is equal to the partial pressure of the gas plus the vapor pressure of water. This means that the amount of gas collected will be less than the total pressure suggests.
Example 17 |
|
Hydrogen gas is generated by the reaction of nitric acid and elemental iron. The gas is collected in an inverted 2.00 L container immersed in a pool of water at 22°C. At the end of the collection, the partial pressure inside the container is 733 torr. How many moles of H2 gas were generated? Solution We need to take into account that the total pressure includes the vapor pressure of water. According to Table 9.2 "Vapor Pressure of Water versus Temperature", the vapor pressure of water at 22°C is 19.84 torr. According to Dalton’s law of partial pressures, the total pressure equals the sum of the pressures of the individual gases, so
We solve by subtracting:
Now we can use the ideal gas law to determine the number of moles (remembering to convert temperature to kelvins, making it 295 K):
All the units cancel except for mol, which is what we are looking for. So n = 0.0775 mol H2 collected
Test Yourself CO2, generated by the decomposition of CaCO3, is collected in a 3.50 L container over water. If the temperature is 50°C and the total pressure inside the container is 833 torr, how many moles of CO2 were generated? Answer 0.129 mol |
Finally, we introduce a new unit that can be useful, especially for gases. The mole fraction, χi, is the ratio of the number of moles of component i in a mixture divided by the total number of moles in the sample:
(χ is the lowercase Greek letter chi.) Note that mole fraction is not a percentage; its values range from 0 to 1. For example, consider the combination of 4.00 g of He and 5.0 g of Ne. Converting both to moles, we get
The total number of moles is the sum of the two mole amounts:
total moles = 1.00 mol + 0.025 mol = 1.25 mol
The mole fractions are simply the ratio of each mole amount and the total number of moles, 1.25 mol:
The sum of the mole fractions equals exactly 1.
For gases, there is another way to determine the mole fraction. When gases have the same volume and temperature (as they would in a mixture of gases), the number of moles is proportional to partial pressure, so the mole fractions for a gas mixture can be determined by taking the ratio of partial pressure to total pressure:
This expression allows us to determine mole fractions without calculating the moles of each component directly.
Example 18 |
|
A container has a mixture of He at 0.80 atm and Ne at 0.60 atm. What are the mole fractions of each component? Solution According to Dalton’s law, the total pressure is the sum of the partial pressures: Ptot = 0.80 atm + 0.60 atm = 1.40 atm
The mole fractions are the ratios of the partial pressure of each component and the total pressure: Again, the sum of the mole fractions is exactly 1. Test Yourself What are the mole fractions when 0.65 atm of O2 and 1.30 atm of N2 are mixed in a container? Answer
|
Food and Drink App: Carbonated BeveragesCarbonated beverages—sodas, beer, sparkling wines—have one thing in common: they have CO2 gas dissolved in them in such sufficient quantities that it affects the drinking experience. Most people find the drinking experience pleasant—indeed, in the United States alone, over 1.5 × 109 gal of soda are consumed each year, which is almost 50 gal per person! This figure does not include other types of carbonated beverages, so the total consumption is probably significantly higher. All carbonated beverages are made in one of two ways. First, the flat beverage is subjected to a high pressure of CO2 gas, which forces the gas into solution. The carbonated beverage is then packaged in a tightly-sealed package (usually a bottle or a can) and sold. When the container is opened, the CO2 pressure is released, resulting in the well-known hiss of an opening container, and CO2 bubbles come out of solution. This must be done with care: if the CO2 comes out too violently, a mess can occur! The second way a beverage can become carbonated is by the ingestion of sugar by yeast, which then generates CO2 as a digestion product. This process is called fermentation. The overall reaction is C6H12O6(aq) → 2C2H5OH(aq) + 2CO2(aq)When this process occurs in a closed container, the CO2 produced dissolves in the liquid, only to be released from solution when the container is opened. Most fine sparkling wines and champagnes are turned into carbonated beverages this way. Less-expensive sparkling wines are made like sodas and beer, with exposure to high pressures of CO2 gas. |
Key Takeaways |
|
Exercises |
|
Additional Exercises |
|
Library Info and Research Help | reflibrarian@hostos.cuny.edu (718) 518-4215
Loans or Fines | circ@hostos.cuny.edu (718) 518-4222
475 Grand Concourse (A Building), Room 308, Bronx, NY 10451
